 , Verónica A. Del Vecchio2
, Verónica A. Del Vecchio2  & Sebastián A. Ausili3 *
& Sebastián A. Ausili3 * 
Research Articles
Clinical management of facial stimulation in cochlear implants
Gabriel S. Rosanigo1  , Verónica A. Del Vecchio2
, Verónica A. Del Vecchio2  & Sebastián A. Ausili3 *
& Sebastián A. Ausili3 * 
1Universidad Nacional de Tres de Febrero, Buenos Aires, Argentina / 2Asociación Argentina de Audiología, Buenos Aires, Argentina / 3University of Miami, Dep. Otolaryngology, Miami, Florida, USA.
 OPEN ACCESS
OPEN ACCESS
PEER REVIEWED
REVIEWS
Abstract
Cochlear implants are currently one of the most effective devices in sensorineural rehabilitation. They offer the possibility of hearing to people with severe to profound hearing loss who derive little benefit from acoustic amplification. However, post-implantation complications can occur, including undesired facial nerve stimulation (FNS). The main symptoms of FNS include involuntary movements of the face and neck, and pain or discomfort during implant use. Sometimes FNS affects a few channels only, but it can also occur in most or even all channels.
Clinical management at present focuses mainly on changing various parameters in device programming. Strategies to reduce FNS range from making changes to the electrical stimulation type and mode to considering reimplantation in complex cases. This article describes FNS and its possible causes, as well as the clinical solutions that are currently available. Finally, new approaches and possible lines of research are discussed.
Keywords
Facial nerve, cochlear implant, electrical stimulation, electrical pulses, stimulation modes
Clinical implications
Cochlear implant (CI) interventions for severe to profound hearing loss achieve excellent results. However, the anatomical proximity of the auditory nerve can cause the electrical fields generated to stimulate the facial nerve. This adverse effect has to be managed with the clinical tools that are available at present. This article provides a review of the literature specifically to:
- investigate the actual incidence of facial nerve stimulation (FNS) in CI users.
- describe the clinical strategies available to manage this aberrant stimulation.
- discuss the most relevant current lines of research
The aim of this research is to guide and update audiology professionals in FNS management in their clinical practice.
Received: 16.09.2022 Revised: 15.10.2022 Accepted: 2.11.2022 Published: 15.11.2022
Edited by:
Miriam Marrufo-Pérez
Universidad de Salamanca, España.
Reviewed by:
Clemencia Baron de Otero
Instituto Nacional de Otología García Gómez, Colombia.
Jose Manuel Gorospe
Gerencia Regional de Salud de Castilla y León, Universidad de Salamanca, España.
Isabel Olleta
Centro de Audiología y Logopedia Isabel Olleta, España.
Introduction
Less than 50 years have passed since the first case reports were published of auditory nerve electrical stimulation using an implantable device (Bergstrom, 1975). By the start of the 1980s, there was already clear evidence of benefit for adult implant recipients (House & Berliner, 1982) and therefore children also started receiving CIs (Eisenberg & House, 1982). Now, most adults with post-ling hearing loss achieve 70% open-specch perception in silence (Lenarz, 2018) and 80% are able to use the telephone (Lenarz et al., 2022). At the start of the 1990s, the advantage of CI over hearing aids became evident when evaluating speech perception skills acquired by profoundly deaf children (Geers & Moog, 1991). We now know that children who receive implants at an early age (before 12 months) can acquire similar levels of language development to normal-hearing children (Ching et al., 2014) and that the best outcomes are achieved by providing access to sound in both ears (Sharma et al., 2020). As a result of this success, there are now more than one million CI users globally (Fan-Gang, 2022).
Although cochlear implantation carries minimal risk (House & Berliner, 1982), some problems still persist, decreasing benefits and, in some cases, even making CI use impossible. One of these adverse effects is non-auditory stimulation in the form of facial nerve stimulation (FNS). The problem has been reported since the start of multichannel device implantations (Cohen et al., 1988) and affects men and women equally (Smullen et al., 2005), as well as children and adults (Alzhrani, 2020).
This undesired stimulation occurs when the electrical field generated by any of the electrodes spreads and reaches a segment of the facial nerve (Kim et al., 2018; see Figure 1), causing discomfort and involuntary ipsilateral movements of the face, around the eye, mouth, nasolabial fold and forehead (Kelsall et al., 1997). Although FNS usually manifests when the CI is first activated, it can also develop after years of use (Berrettini et al., 2011). Often, FNS worsens over time, affecting more stimulation channels and being triggered by low charge levels. One retrospective analysis found that FNS can progress until speech perception in silence drops below acceptable levels due to the need to deactivate multiple channels, even after CI has been reimplanted (Polak et al., 2006).
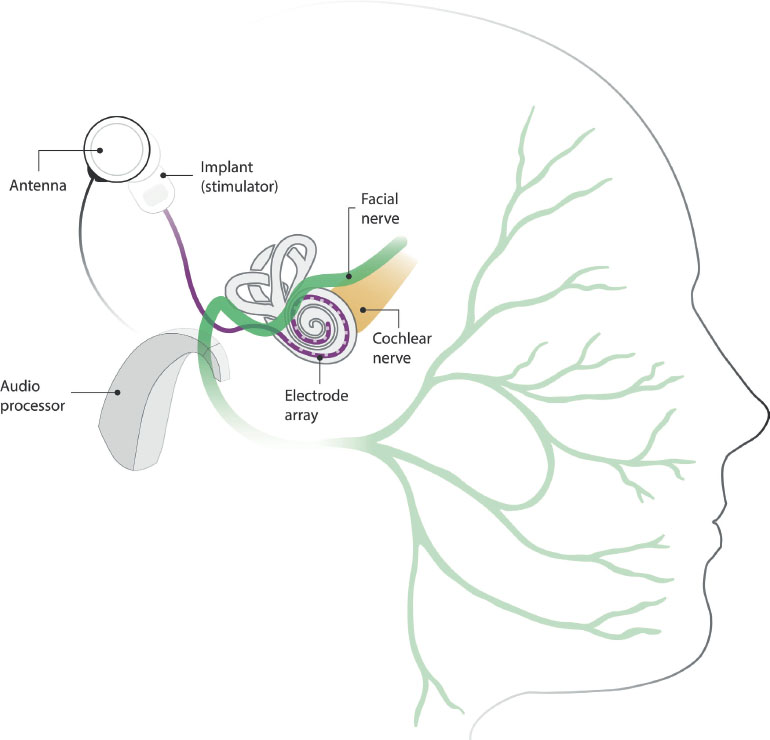
Figure 1. Illustration of a cochlear implant and its anatomical proximity to the facial nerve. The audio processor transmits the signal to the implanted device via its antenna. The electrode array, located in the cochlea, stimulates the cochlear nerve through electrical fields generated in the active electrodes. Anatomical proximity between the electrical stimulation and the facial nerve may result in undesired nerve stimulation.
FNS incidence and associations with anatomy and aetiology
One recent study reviewed 37 publications (representing 5936 CI users) and found that the overall reported FNS rate was approximately 6% (Van Horn et al., 2020). This number increases significantly in patients with otosclerosis, cochlear malformation, or ossification, reaching almost 50% in these population groups (Weber et al., 1998). Otosclerosis reduces impedance and increases conductivity shunting the current through the bone, which allows the current to reach the facial nerve (Seyyedi et al., 2013). Recently, a study of 351 otosclerotic ears reported 10.5% of FNS incidence (Assiri et al., 2022). Moreover, other authors have also observed that patients with otosclerosis were more likely to experience FNS compared with those with other cochlear pathologies (28.4% vs. 3.5%, respectively; Van Horn et al., 2020).
In patients with malformations, FNS is likely to affect most electrodes (Kim et al., 2018). Malformations of the facial nerve itself also increase the probability of aberrant CI stimuli (Chen et al., 2021). Likewise, temporal bone fractures may increase the incidence of FNS (Espahbodi et al., 2015).
Another predisposing factor for the onset of FNS may be the proximity between the labyrinthine segment of the facial nerve and the first turn of the cochlea (Erixon et al., 2009; Kasetty et al., 2019; Aljazeeri et al., 2021). This factor is corroborated by studies that found that electrodes nearest to this segment are most likely to trigger undesired stimulation (Smullen et al., 2005; Kelsall et al., 1997). One research group investigated anatomical characteristics that might predict the risk for FNS onset in a blinded study using computed tomography (Hatch et al., 2017). The authors found significant anatomical differences in the separation between the cochlea and the labyrinthine segment of the facial nerve in CI users with FNS. It is also believed that the bony separation between the scala tympani of the cochlea and the facial nerve may be eroded under the pressure exerted by the electrode (Smullen et al., 2005). Although these findings are encouraging, further studies are still needed in this research line to improve and increase their predictive power.
One animal study reported low impedance levels in the basal electrodes, which may allow for greater current spread (Niparko et al., 1991). An association was also proposed between FNS and the dimensions of the bony cochlear nerve canal and the internal auditory canal in a study that found both structures to be narrower when FNS was present (Rah et al., 2016). Furthermore, a correlation was found between a narrower vestibular aqueduct and a shorter internal auditory canal in individuals with FNS (Kamogashira et al., 2017).
Associations between FNS and device design
A systematic review by Van Horn et al. (2020) found that lateral wall electrodes significantly increased the incidence of FNS compared with perimodiolar electrodes (15.7% vs. 4.4%, respectively). Similar findings have been reported by other authors (Burck et al., 2022; Ahn et al., 2009; Matterson et al., 2007; Battmer et al., 2006; Marshall et al., 2005). However, some studies have found that FNS incidence is unaffected by electrode array type (Abdelhamed, 2019). Some authors report that lateral wall electrodes tend to be used more in malformations, and therefore electrode design is no longer a significant factor when comparing anatomically normal cochleae (Ahn et al., 2009).
Closer proximity of the electrodes to the modiolus, together with correct positioning, may reduce the risk of FNS (Battmer et al., 2006). The proximity of the electrode to the central axis of the cochlea tends to reduce the required perceptual current levels, which in turn helps to maintain electrical stimulation below the FNS threshold (Seyyedi et al., 2013). Furthermore, computational models showing electrical field spread suggest that full-band electrodes are the most likely to cause FNS, followed by half-band electrodes and finally, plate electrodes (Frijns et al., 2009).
Clinical solutions to manage FNS
When the FNS threshold is lower than the user's comfort hearing level, it negatively affects auditory performance and audiologists have to mitigate the problem by adapting the CI fitting. One solution is to reduce the current level of the channels or even deactivate them, which usually worsens the user’s performance. When these actions have to be taken, some CI users may stop using the device due to lack of benefit (Nassiri et al., 2018). However, these programming changes are effective in many cases (Smullen et al., 2005; Pires et al., 2018).
These alternatives are an option when FNS is present in only a few channels but are not the best solution in cases where most of the CI electrode channels are affected. Depending on the degree of FNS severity and considering the lack of clinical resources for FNS management, reimplantation may be considered (Battmer et al., 2006; Walia et al., 2021). Unfortunately, device replacement sometimes leaves the problem unsolved, and FNS can persist even after more than one reimplantation (Morris et al., 2004). Although a device change may be ineffective in itself, it makes available new feeting tools to manage the problem (Polak et al., 2006).
Pulse morphology
Another alternative available to avoid FNS is to modify pulse morphology (Fig. 2). Increasing the pulse duration or using triphasic instead of biphasic pulses are strategies to increase sound perception without FNS.
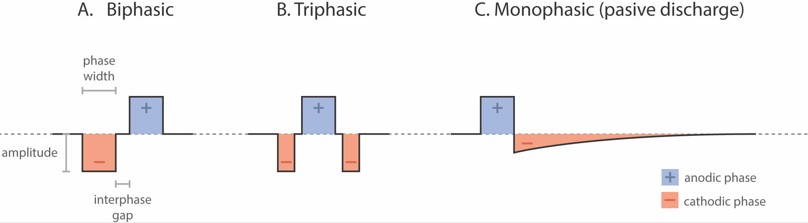
Figure 2. Stimulation pulse shape options in cochlear implants. (A) Biphasic pulse; (B) Triphasic pulse; (C) Monophasic with passive discharge pulse. Depending on the pulse shape used, the parameters that can be modified are amplitude, phase width, and interphase gap.
Increasing the total pulse width delivers a higher charge or energy with a reduced pulse amplitude (Bahmer et al., 2017). The effectiveness of this strategy has been repeatedly demonstrated in FNS management (Polak et al., 2006; Pires et al., 2018; Sefien & Hamada, 2019).
Biphasic pulses generated by CIs usually consist of two opposing polarities of equal duration and amplitude: a negative or cathodic phase and a positive or anodic phase (Fig. 2A). Triphasic pulses have two cathodic phases of the same duration and amplitude, and between the two, an anodic phase with opposite polarity of the same amplitude but double the duration (Fig. 2B). As a result, pulses are charge-balanced (Braun et al., 2019). It was shown that triphasic pulses can benefit CI users with or without FNS (Bonnet et al., 2004). Schatzer et al., (2014) studied the use of triphasic pulses to selectively stimulate the auditory nerve in cases of FNS. Since then, many studies have demonstrated that triphasic pulses help resolve this complication (Schatzer et al., 2014; Bahmer & Baumann, 2016; Bahmer et al., 2017; Braun et al., 2019; Alhabib et al., 2021). Although triphasic phases need a higher current level than biphasic pulses to get the same loudness perception level (Bahmer et al., 2016), the most comfortable levels achieved without eliciting FNS are significantly higher (Bahmer & Baumann, 2016; Bahmer et al., 2017, Braun et al., 2019; Alhabib et al., 2021). This strategy improves auditory performance in CI users with FNS, enhancing their speech discrimination (Bahmer & Baumann, 2016; Alhabib et al., 2021).
Another less common FNS management strategy is to increase the interphase gap, which is the time that elapses between the positive and negative phases of each pulse (Fig. 2A). Cochlear implant manufacturers set their own default gap, but some allow the gap to be modified using the fitting software. A longer interphase gap allows for a lower threshold and most-comfortable levels, reducing the likelihood of FNS (Pieper et al., 2020). In addition to biphasic and triphasic pulses, a monophasic passive discharge pulse also exists (Fig. 2C), consisting of an anodic phase followed by a cathodic phase, which is a passive discharge due to a capacitive effect (Fig. 2C). This pulse shape produces less current spread than the widely used biphasic pulses (Zellhuber et al., 2022). Monophasic passive discharge pulses also need less current than triphasic pulses to reach comfortable levels (Macherey et al., 2006; Macherey et al., 2008; Carlyon et al., 2013).
Stimulation modes
Most cochlear implants use a monopolar stimulation mode in which active intracochlear electrodes create an electrical field referenced to an extracochlear electrode (Fig. 3A). This reference electrode may be located inside or outside the implant body, under the Temporal muscle, with its own contact. The wide current spread – required because of the distance between the intra- and extracochlear electrodes – increases the risk of the current flow reaching the facial nerve.
To reduce this spread and achieve more selective stimulation, a bipolar (BP) mode has been introduced (Fig. 3B). Bipolar stimulation uses one of the intracochlear electrodes as reference. The distance between the active and reference electrodes can be set, whereby BP+1 uses a neighbour electrode and BP+n skips n electrodes in between. Note that this configuration is limited by the total number of electrodes. By limiting the electrical field spread with bipolar stimulation, FNS can be significantly reduced. Computational modelling has shown that this stimulation mode can substantially increase in FNS thresholds (van der Westhuizen et al., 2022).
Another type of stimulation that is also effective for FNS is multi-mode grounding, also known as mixed-mode grounding (Fig. 3C). In multi-mode grounding, all intracochlear electrodes are used for grounding except the channel to be stimulated. This design discharges around 80% of the current inside the cochlea (Dang, 2017), thus reducing current spread.
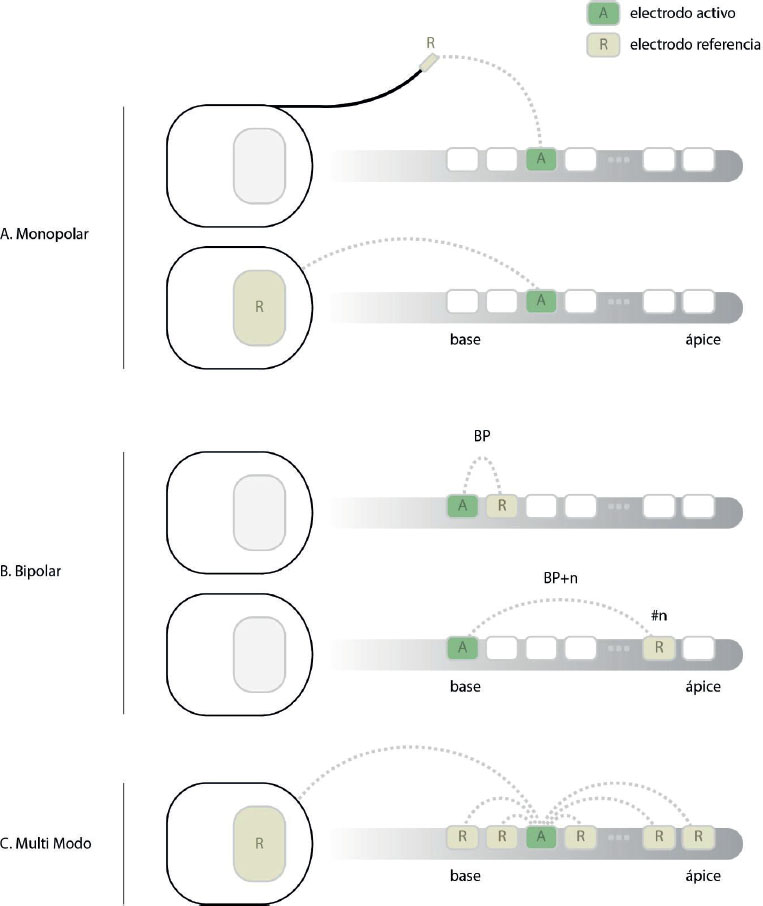
Figure 3. Illustration of stimulation modes in cochlear implants. (A) Monopolar stimulation with integrated or external reference stimulation; (B) Bipolar stimulation; (C) Multi-mode grounding.
The multi-mode grounding system also modifies the pulse phase width (stimulation duration) to increase the charge delivered and incorporates monophasic passive discharge pulses. (Fig. 2C). The combination of these variables has been found to effectively address the problem of FNS (Zellhuber et al., 2022; Eitutis et al., 2022). This set-up appears to be an excellent clinical strategy, but further studies are needed to quantify the contribution of each variable and their actual effect on reducing FNS.
Discussion
Cochlear implants have been proven to effectively improve the quality of life of millions of individuals. However, some problems persist that reduce users’ auditory performance, including undesired facial nerve stimulation.
Some disagreement remains about the precise incidence of FNS since multiple interrelated factors are involved. In any case, solving or alleviating this complication is of utmost importance because users’ expectations of their CI may be unmet, and they may even stop using the device.
While the problem can be managed in various ways, there is no single or fully effective approach to solve FNS. Current solutions available to clinicians include reprogramming CI parameters. Several research studies have found that changes to the pulse shape and characteristics effectively reduce FNS.
Two such changes that have improved the situation for users with FNS are increasing the pulse width while maintaining amplitude and lengthening the interphase gap. The two most effective, novel alternatives are triphasic pulse and monophasic passive discharge pulse stimulation systems.
Another approach is to limit electrical field spread by modifying the monopolar, bipolar or multi-mode stimulation mode. Although studies show that changing these modes is beneficial for FNS, further work is needed to fully optimize the outcome of such changes. In current configurations, reprogramming the mode can lead to a change in the CI coding strategy, with which could affect the benefit that the user have with the CI. Bipolar stimulation, for example, reduces the final number of active electrodes, which could worsen users’ auditory performance. Furthermore, the higher electrical charge required in bipolar mode increases battery consumption. All these aspects must be considered when addressing FNS.
Not all the options mentioned in this review are available in all CI models or offered by all manufacturers. Clinicians must decide which is the best option for each device and each user. Often, a combination of approaches is a good strategy to reduce or totally eliminate FNS.
New research and ways of stimulating the auditory nerve may also provide solutions to reduce FNS. Landsberger et al. (2022) have demonstrated the possibility of perceiving lower frequencies with short electrodes by placing the extracochlear reference electrode in the cochlear apex. This novel configuration redirects the electrical field. Although this study did not address FNS, redirecting the electrical field could reduce the current flow to the facial nerve. Badenhorst et al. (2021) used three-dimensional computer modelling for diagnostic purposes to study the relationship between electrical fields and the facial nerve. This new approach may provide more information about FNS in a format that can be adapted on a case-by-case basis. In addition, the study showed that computer modelling can be used to measure cochlear bone lining conductivity and predict which channels are most prone to FNS. These promising results may help bring computer modelling into clinical use for assessing and programming CIs in users with FNS.
Clinicians should remember that all the resources available to manage this aberrant stimulation may have an impact on users’ hearing performance and quality of life. Users may reject many of these changes because they entail adapting to a new way of listening. Counselling plays a crucial role in this setting. Users must receive clear information to understand the reasons for making these changes. The starting point here is to seek the best outcome by combining existing technical-clinical tools and offering CI users all possible support.
Conclusions
Cochlear implant electrical stimulation can cause FNS, a complication that varies depending on the aetiology and the anatomical characteristics of the inner ear, as well as the characteristics of the implant itself. Current clinical practice allows clinicians to manage FNS by decreasing charge level, deactivating channels and changing pulse shape and stimulation modes. Although new tools are available, this undesired stimulation cannot always be eliminated. Further studies are needed to quantify the effectiveness of these tools, as well as to develop new technologies to eradicate the problem.
References
Abdelhamed, M. Z. (2019). Evaluation of the Triphasic Pulse Stimulation in Eliminating Facial Nerve Stimulation in Cochlear Implant Recipients. Glob J Oto, 19(2); https://doi.org/10.19080/GJO.2019.19.556007.
Ahn JH, Oh SH, Chung JW, Lee KS. (2009). Facial nerve stimulation after cochlear implantation according to types of Nucleus 24-channel electrode arrays. Acta Otolaryngol, 129(6):588–91. https://doi.org/10.1080/00016480802325965.
Alhabib S, Abdelsamad Y, Yousef M & Alzhrani F. (2021). Performance of cochlear implant recipients fitted with triphasic pulse patterns. Eur Arch Otorhinolaryngol, 278(9):3211–3216. https://doi.org/10.1007/s00405-020-06382-0.
Aljazeeri IA, Khurayzi T, Al-Amro M, Alzhrani F, Alsanosi A. (2021). Evaluation of computed tomography parameters in patients with facial nerve stimulation post-cochlear implantation. Eur Arch Otorhinolaryngol, 278(10):3789–3794. https://doi.org/10.1007/s00405-020-06486-7.
Alzhrani F, Halawani R, Basodan S, Hudeib R. (2020). Investigating facial nerve stimulation after cochlear implantation in adult and pediatric recipients. Laryngoscope, 131(2):374–379. https://doi.org/10.1002/lary.28632.
Assiri M, Khurayzi T, Alshalan A, Alsanosi A. (2022). Cochlear implantation among patients with otosclerosis: a systematic review of clinical characteristics and outcomes. Cochlear implantation among patients with otosclerosis: a systematic review of clinical characteristics and outcomes, 279(7):3327–3339. https://doi.org/10.1007/s00405-021-07036-5.
Badenhorst W, Hanekom T, Gross L, Hanekom JJ. (2021). Facial nerve stimulation in a post-meningitic cochlear implant user: using computational modelling as a tool to probe mechanisms and progression of complications on a case-by-case basis. Cochlear Implants Int, 22(2):68–79. https://doi.org/10.1080/14670100.2020.1824431.
Bahmer A, Adel Y, Baumann U. (2017). Preventing Facial Nerve Stimulation by Triphasic Pulse Stimulation in Cochlear Implant Users: Intraoperative Recordings. Otol Neurotol, 38(10):e438–e444. https://doi.org/10.1097/MAO.0000000000001603.
Bahmer A, Baumann U. (2016). The underlying mechanism of preventing facial nerve stimulation by triphasic pulse stimulation in cochlear implant users assessed with objective measure. Otol Neurotol, 37(9):1231–7. https://doi.org/10.1097/MAO.0000000000001156.
Battmer R, Pesch J, Stöver T, Lesinski-Schiedat A, Lenarz M, Lenarz T. (2006). Elimination of facial nerve stimulation by reimplantation in cochlear implant subjects. Otol Neurotol, 27(7):918–22. https://doi.org/10.1097/01.mao.0000235374.85739.c6.
Bergstrom, L. (1975). Some pathologies of sensory and neural hearing loss. Can J Otolaryngol Suppl, 2:1–28.
Berrettini S, Vito de A, Bruschini L, Passetti S, Forli F. (2011). Facial nerve stimulation after cochlear implantation: our experience. Acta Otorhinolaryngol Ital, 31:11–6.
Bonnet RM, Frijns JH, Peeters S, Briaire JJ. (2004). Speech recognition with a cochlear implant using triphasic charge-balanced pulses. Acta Otolaryngol, 124(4):371–5. https://doi.org/10.1080/00016480410031084.
Braun K, Walker K, Surth W, Lowenheim H & Tropitzsch A. (2019). Triphasic Pulses in Cochlear Implant Patients With Facial Nerve Stimulation. Starnberg : Otol Neurotol, 40(10):1268–1277. https://doi.org/10.1097/MAO.0000000000002398.
Burck I, Helal RA, Naguib NNN, Nour-Eldin NA, Scholtz JE, Martin S, Leinung M, Helbig S, Stöver T, Lehn A, Vogl TJ. (2022). Postoperative radiological assessment of the mastoid facial canal in cochlear implant patients in correlation with facial nerve stimulation. Eur Radiol, 32(1):234–242. https://doi.org/10.1007/s00330-021-08128-w.
Carlyon RP, Deeks JM, Macherey O. (2013). Polarity effects on place pitch and loudness for three cochlear-implant designs and at different cochlear sites. J Acoust Soc Am, 134(1):503–9. https://doi.org/10.1121/1.4807900.
Chen J, Chen B, Zhang L, Li Y. (2021). Severe and persistent facial nerve stimulation after cochlear implantation in a patient with cochlear-facial dehiscence: a case report. J Int Med Res, 49(11):3000605211057823. https://doi.org/10.1177/03000605211057823.
Ching TY, Day J, Van Buynder P, Hou S, Zhang V, Seeto M, Burns L, Flynn C. (2014). Language and speech perception of young children with bimodal fitting or bilateral cochlear implants. Cochlear Implants Int, 15 Suppl 1(0 1):S43–6. https://doi.org/10.1179/1467010014Z.000000000168.
Cohen NL, Hoffman RA, Stroschein M. (1988). Medical or surgical complications related to the nucleus multichannel cochlear implant. Ann Otol Rhinol Laryngol, 97:8–13; https://doi.org/10.1177/00034894880975s202.
Dang, K. (2017). Electrical conduction models for cochlear implant stimulation. HAL Open Science.
Eisenberg LS, House WF. (1982). Initial experience with the cochlear implant in children. Ann Otol Rhinol Laryngol Suppl, (2 Pt 3):67–73.
Eitutis ST, Carlyon RP, Tam YC, Salorio-Corbetto M, Vanat Z, Tebbutt K, Bardsley R, Powell HRF, Chowdhury S, Tysome JR, Bance ML. (2022). Management of Severe Facial Nerve Cross Stimulation by Cochlear Implant Replacement to Change Pulse Shape and Grounding Configuration: A Case-series. Otol Neurotol, 43(4):452–459. https://doi.org/10.1097/MAO.0000000000003493.
Erixon E, Högstorp H, Wadin K, Rask-Andersen H. (2009). Variational anatomy of the human cochlea: implications for cochlear implantation. Otol Neurotol, 0(1):14–22. https://doi.org/10.1097/MAO.0b013e31818a08e8.
Espahbodi M, Sweeney AD, Lennon KJ, Wanna GB. (2015). Facial nerve stimulation associated with cochlear implant use following temporal bone fractures. Am J Otolaryngol, 36(4):578–582; https://doi.org/10.1016/j.amjoto.2015.04.003.
Fan-Gang, Z. (2022). Celebrating the one millionth cochlear implant. JASA Express, Lett. 2. 077201; https://doi.org/10.1121/10.0012825.
Frijns JH, Kalkman RK, Briaire JJ. (2009). Stimulation of the facial nerve by intracochlear electrodes in otosclerosis: a computer modeling study. Otol Neurotol, 30(8):1168–74. https://doi.org/10.1097/MAO.0b013e3181b12115.
Geers AE, Moog JS. (1991). Evaluating the benefits of cochlear implants in an education setting. Am J Otol, 12 Suppl:116–25.
Hatch JL, Rizk HG, Moore MW, Camposeo EE, Nguyen SA, Lambert PR, Meyer TA, McRackan TR. (2017). Can Preoperative CT Scans Be Used to Predict Facial Nerve Stimulation Following CI? Otol Neurotol, 38(8):1112–1117. https://doi.org/10.1097/MAO.0000000000001497.
House WF, Berliner KI. (1982). The cochlear implant. Otolaryngol Clin North Am, 15(4):917–23.
Kamogashira T, Iwasaki S, Kashio A, Kakigi A, Karino S, Matsumoto Y, Yamasoba T. (2017). Prediction of Intraoperative CSF Gusher and Postoperative Facial Nerve Stimulation in Patients With Cochleovestibular Malformations Undergoing Cochlear Implantation Surgery. Otol Neurotol, 38(6):e114–e119. https://doi.org/10.1097/MAO.0000000000001440.
Kasetty VM, Zimmerman Z, King S, Seyyedi M. (2019). Comparison of Temporal Bone Parameters before Cochlear Implantation in Patients with and without Facial Nerve Stimulation. J Audiol Otol, 23(4):193–196. https://doi.org/10.7874/jao.2019.00129.
Kelsall DC, Shallop JK, Brammeier TG, Prenger EC. (1997). Facial nerve stimulation after Nucleus 22-channel cochlear implantation. Am J Otol, 18(3):336–341.
Kim YR, Yoo MH, Lee JY, Yang CJ, Park JW, Kang BC, Kang WS, Ahn JH, Chung JW, Park HJ. (2018). Characteristics and pathogenesis of facial nerve stimulation after cochlear implant surgeries: A single-centre retrospective analysis from 1151 patients. Clin Otolaryngol, 43(5):1396–1400. https://doi.org/10.1111/coa.13153.
Landsberger DM, Stupak N, Spitzer ER, Entwisle L, Mahoney L, Waltzman SB, McMenomey S, Friedmann DR, Svirsky MA, Shapiro W, Roland JT Jr. (2022). Stimulating the Cochlear Apex Without Longer Electrodes: Preliminary Results With a New Approach. Otol Neurotol, 43(5):e578–e581. https://doi.org/10.1097/MAO.0000000000003529.
Lenarz T, Büchner A, Illg A. (2022). Cochlear Implantation: Concept, Results Outcomes and Quality of Life. Laryngorhinootologie, 101(S 01):S36-S78. English, German. https://doi.org/10.1055/a-1731-9321.
Lenarz, T. (2018). Cochlear implant - state of the art. GMS Curr Top Otorhinolaryngol Head Neck Surg, 16:Doc04. https://doi.org/10.3205/cto000143.
Macherey O, Carlyon RP, van Wieringen A, Deeks JM, Wouters J. (2008). Higher sensitivity of human auditory nerve fibers to positive electrical currents. J Assoc Res Otolaryngol, 9(2):241–51. https://doi.org/10.1007/s10162-008-0112-4.
Macherey O, van Wieringen A, Carlyon RP, Deeks JM, Wouters J. (2006). Asymmetric pulses in cochlear implants: effects of pulse shape, polarity, and rate. J Assoc Res Otolaryngol, 7(3):253–66. https://doi.org/10.1007/s10162-006-0040-0.
Marshall AH, Fanning N, Symons S, Shipp D, Chen JM, Nedzelski JM. (2005). Cochlear implantation in cochlear otosclerosis. Laryngoscope, 115(10):1728–33. https://doi.org/10.1097/01.mlg.0000171052.34196.ef.
Matterson AG, O'Leary S, Pinder D, Freidman L, Dowell R, Briggs R. (2007). Otosclerosis: selection of ear for cochlear implantation. Otol Neurotol, 28(4):438–46. https://doi.org/10.1097/MAO.0b013e31803115eb.
Morris DP, Maessen H, Creaser C, van Wijhe R, Bance M. (2004). Refractory severe facial nerve cross-stimulation and loss of auditory sensation after ten years of uneventful cochlear implant use. A rare and challenging case. Cochlear Implants Int, 5(3):117–24. https://doi.org/10.1179/cim.2004.5.3.117.
Nassiri, A. M., Yawn, R. J., Dedmon, M. M., O’Connell, B. P., Holder, J. T., Haynes, D. S., & Rivas, A. (2018). Facial nerve stimulation patterns associated with cochlear implantation in labyrinthitis ossificans. Otology and Neurotology, 39(10), e992–e995. https://doi.org/10.1097/MAO.0000000000002028
Niparko JK, Oviatt DL, Coker NJ, Sutton L, Waltzman SB, Cohen NL. (1991). Facial nerve stimu-lation with cochlear implant. Otolaryngol Head Neck Surg. 104:826–830. 104(6):826–30. https://doi.org/10.1177/019459989110400610
Pieper SH, Brill S, Bahmer A. (2020). Loudness Perception and Dynamic Range Depending on Interphase Gaps of Biphasic Pulses in Cochlear Implants. Ear Hear, 41(5):1251–1257. https://doi.org/10.1097/AUD.0000000000000843.
Pires JS, Melo AS, Caiado R, Martins JH, Elói Moura J, Silva LF. (2018). Facial nerve stimulation after cochlear implantation: Our experience in 448 adult patients. Cochlear Implants Int, 19(4):193–197. https://doi.org/10.1080/14670100.2018.1452561.
Polak M, Ulubil SA, Hodges AV, Balkany TJ. (2006). Revision cochlear implantation for facial nerve stimulation in otosclerosis. Arch Otolaryngol Head Neck Surg, 132(4):398–404. https://doi.org/10.1001/archotol.132.4.398.
Rah YC, Yoon YS, Chang MY, Lee JY, Suh MW, Lee JH, Oh SH, Chang SO, Park MK. (2016). Facial nerve stimulation in the narrow bony cochlear nerve canal after cochlear implantation. Laryngoscope, 126(6):1433–9. https://doi.org/10.1002/lary.25655.
Schatzer R. (2014). Selective suppression of facial nerve activation in CI patients with tripha sic stimulation. 13th International Conference on Cochlear Implants and Other Implantable Auditory Implants. Munich
Sefien I, Hamada S. (2019). Facial Nerve Stimulation as a Complication of Cochlear Implantation. Indian J Otolaryngol Head Neck Surg, 71(4):474–479. https://doi.org/10.1007/s12070-019-01649-3.
Seyyedi M, Herrmann BS, Eddington DK, Nadol JB. (2013). The pathologic basis of facial nerve stimulation in otosclerosis and multi-channel cochlear implantation. Otol Neurotol, 34:1603–9; https://doi.org/10.1097/MAO.0b013e3182979398.
Sharma SD, Cushing SL, Papsin BC, Gordon KA. (2020). Hearing and speech benefits of cochlear implantation in children: A review of the literature. Int J Pediatr Otorhinolaryngo, 133:109984. https://doi.org/10.1016/j.ijporl.2020.109984.
Smullen JL, Polak M, Hodges AV, Payne SB, King JE 3rd, Telischi FF, Balkany TJ. (2005). Facial nerve stimulation after cochlear implantation. Laryngoscope, 115(6):977–82. https://doi.org/10.1097/01.
van der Westhuizen J, Hanekom T, Hanekom JJ. (2022). Apical Reference Stimulation: A Possible Solution to Facial Nerve Stimulation. Ear Hear, 43(4):1189–1197. https://doi.org/10.1097/AUD.0000000000001170.
van Horn A, Hayden C, Mahairas AD, Leader P, Bush ML. (2020). Factors Influencing aberrant facial nerve stimulation following cochlear implantation: a systematic review and meta-analysis. Otol Neurotol., 41(8):1050–1059; https://doi.org/10.1097/MAO.0000000000002693.
Walia A, Shew MA, Ortmann AJ, Buchman CA, Herzog JA. (2021). Hearing Preservation After Cochlear Reimplantation Using Electrocochleography: A Case Report. Laryngoscope, 131(10):2348–2351. https://doi.org/10.1002/lary.29734.
Weber BP, Dillo W, Dietrich B, Maneke I, Bertram B, Lenarz T. (1998). Pediatric cochlear implantation in cochlear malformations. Am J Otol, 19(6):747–53.
Zellhuber N, Helbig R, James P, Bloching M, Lyutenski S. (2022). Multi-mode grounding and monophasic passive discharge stimulation avoid aberrant facial nerve stimulation following cochlear implantation. Clin Case Rep., 10(2):e05360. https://doi.org/10.1002/ccr3.5360.
Conflicts of interest
The authors declare no conflict of interest. They declare no commercial or financial relationships during the conduct of this review that could be construed as a potential conflict of interest.
Author contributions:
GR and VD conceived, drafted, reviewed and edited the manuscript and visualized its presentation; SA conceived, supervised, wrote, reviewed and edited the manuscript.
How to cite:
Rosanigo, G., Del Vecchio, V., & Ausili, S. (2022).
Abordaje clínico de la estimulación facial en
implantes cocleares. Auditio, 6, e90.
https://doi.org/10.51445/sja.auditio.vol6.2022.0090
Correspondence
*Sebastián A. Ausili
1120 NW 14th St, Miami, FL 33136, USA
email: s.ausili@miami.edu
Editorial Office
Correction: Emma Goldsmith.
Translation: Tomás Pérez Pazos.
Producción: Publicaciones Académicas.