Research Articles
Do complications of percutaneous osseointegration justify a switch to transcutaneous devices? A retrospective longitudinal study on complications
Marta Núñez-Gutiérrez1* , Juan Castro-Jiménez1
, Juan Castro-Jiménez1 , Francisco Fernández-Nogueras Jiménez1, Juan M. Espinosa-Sánchez2, 3, 4, 5
, Francisco Fernández-Nogueras Jiménez1, Juan M. Espinosa-Sánchez2, 3, 4, 5 , Juan García-Valdecasas2, 6
, Juan García-Valdecasas2, 6
1 Otorhinolaryngology Department, Hospital Universitario Virgen de las Nieves, Granada, Spain / 2 Otorhinolaryngology Department, Instituto de Investigación Biosanitaria ibs.GRANADA, Hospital Universitario Virgen de las Nieves, Granada, Spain / 3 Otology and Neurotology Group CTS495, Genomic Medicine Department, GENYO, Centro Pfizer-Universidad de Granada-Junta de Andalucía de Genómica e Investigación Oncológica, Granada, Spain / 4 Otolaryngology Division, Department of Surgery, Universidad de Granada, Granada, Spain / 5 Neurosensory Pathology Programme, Centro de Investigación Biomédica en Red en Enfermedades Raras (CIBERER), Madrid, Spain / 6 Clínica SENT, Granada, Spain.
 OPEN ACCESS
OPEN ACCESS
PEER REVIEWED
ORIGINAL RESEARCH
DOI: 10.51445/sja.auditio.vol7.2023.0089
Abstract
Introduction: Percutaneous bone conduction implants are the standard osseointegration model used to provide an alternative treatment option for conductive and mixed hearing loss. In recent years, the indications for these implants have increased, despite concerns about their use because of local complications.
The aim of this study was to describe the complications of percutaneous osseointegrated devices implanted at our hospital.
Material and methods: A retrospective longitudinal study was conducted in 57 consecutive patients who received a Baha®-type percutaneous bone conduction implantation. The main clinical indications were chronic otitis media and ear malformations. Local peri-implant complications were described using the Holgers classification.
Results: After a mean follow-up of 12 months (range: 4-48 months), 26.31% of patients had some type of peri-implant soft tissue complication. Only one patient (1.75%) had major reactions requiring removal of the implant. Complications in the paediatric age group were notably higher, affecting 42.85% of the children.
Conclusions: The local complication rate in our study was at the lower end of the range described in the literature, which reports a very wide range of rates, even reaching 70%. Most complications are minor and resolve with topical antibiotic treatment. However, the rate is higher in children.
Keywords
Percutaneous bone conduction implant, local complications, Holgers, BAHA, Baha®.
Clinical Implications
Major peri-implant skin reactions are rare. Switching to transcutaneous osseointegration is not justified for this reason alone. The most appropriate bone-anchored device should be selected on a patient-by-patient basis, considering primarily the audiological indication and hearing loss at high frequencies in particular, since the use of passive transcutaneous devices may hinder discrimination. The surgical team’s experience and financial considerations must also be taken into account.
Received: 15.09.2022 Reviewed: 05.12.2022 Acepted: 16.03.2023 Published: 02.11.2023
Edited by:
Sebastián Ausili
University of Miami.
Revisado por:
María José Lavilla Martín de Valmaseda
Hospital Clínico Universitario Lozano Blesa, España.
Marta Herrero Romero
Hospital Universitario Quirón, España.
Mario Zernotti
Universidad Católica de Córdoba, Argentina.
Javier Gavilán
Hospital Universitario de la Paz, España.
Introduction
In recent years, bone conduction implants have received increasing acceptance and interest (Lavilla et al., 2019). They are an effective treatment option in patients with conductive, mixed or profound sensorineural hearing loss with normal contralateral hearing and are increasingly used for both current and new indications (Lavilla Martín de Valmaseda et al., 2019; Yuen et al., 2009).
Bone conduction implants are semi-implantable medical devices consisting of an external processor, a microphone and an amplifier. They transmit sound to the cochlea, allowing intracochlear fluids to vibrate, bypassing the outer and middle ear (Lavilla Martín de Valmaseda et al., 2019). The difference between percutaneous and transcutaneous bone conduction implants lies in the way the external processor and the implant are connected (Figure 1). Percutaneous systems have a direct connection between the two, whereby the implant emerges through the skin, while transcutaneous systems involve communication through intact skin. Transcutaneous conduction systems are divided into passive or skin-drive devices (where vibrations are transmitted through intact skin by magnets) and active or direct-drive devices (where vibrations are generated directly by the implant; Lavilla Martín de Valmaseda et al., 2019).
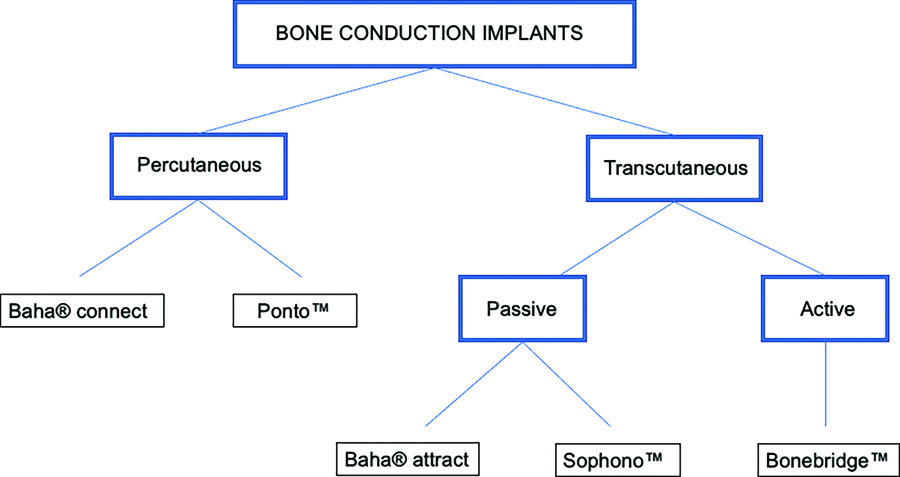
Figure 1. Types of bone conduction implants
Passive transcutaneous devices consist of a titanium implant that is placed under the skin, with a baseplate that protrudes under the skin. Sound is transmitted through the intact skin from a passive transducer to the baseplate, whereas active transcutaneous devices are characterised by the vibration being generated directly by the implant after receiving the correctly processed sound (Reinfeldt et al., 2015).
Osseointegration,as previously describred in the literature is a phenomenon whereby bone tissue grows when it is in contact with a titanium implant, forming a structural and functional connection between the bone and the implant surface that allows sound transmission. (Brånemark et al., 2001; Tjellström et al., 1981). Low-frequency sounds mainly travel through the frontal transmission pathway, but the high-frequency pathway is less well defined (Dufour-Fournier et al., 2022). Bone conduction implants have subsequently been developed that require no titanium-component osseointegration (Bonebridge™; Zernotti and Sarasti, 2015).
Percutaneous bone conduction implants are the standard osseointegration model that provides excellent audiological results (especially at high frequencies), despite some cosmetic concerns. Percutaneous devices may be superior to transcutaneous devices due to the skin damping that occurs in this type of device of up to 5-20 dB for frequencies of 1 to 4 KHz (Verstraeten et al., 2009). Transcutaneous devices provide a better cosmetic solution, which has led to increased use in recent years. Some authors have reported a lower rate of local complications using transcutaneous devices, which also appears to favour their use (Ellsperman et al., 2021). Even so, the force required to hold the magnet in place and achieve good sound transmission can irritate the skin and percutaneous tissues, leading to necrosis in the worst cases (Chen et al., 2017). The most frequent major complications of passive transcutaneous implants are seroma, haematoma, local infection and skin ulcers, which occur in 5.2% of all patients (Cooper et al., 2017).
Surgical techniques in bone conduction device implantation have evolved over the last few decades. Currently, most procedures are performed as single-stage surgery under local or general anaesthesia, using a wide variety of incision approaches (Calon et al., 2018). In paediatric patients, who have thinner cranial bone, osseointegration must occur before inserting the processor; and therefore, a two-stage procedure can be considered (Roman et al., 2011; Verheij et al., 2016). The conventional surgical procedure involves thinning the skin around the implant with the aim of ensuring adequate skin contact with the bone tissue while decreasing the risk of skin overgrowth and local infections (Cass and Mudd, 2010). To try to minimize local complications, new surgical techniques have been developed such as the linear incision technique without soft tissue reduction (den Besten et al., 2016), the punch-only technique, and minimally invasive Ponto surgery (MIPS; Johansson et al., 2017).
Skin condition and cranial thickness may influence the development of skin complications after percutaneous osseointegrated device implantation surgery. Patients with thin or fragile skin or a history of skin diseases such as psoriasis or eczema may be at increased risk for skin irritation, pressure ulcers and post-operative infection (Calon et al., 2018). Another risk factor for device failure in paediatric patients is low socioeconomic status (Kraai et al., 2011).
In addition, greater skull thickness is considered more amenable for implantation, as it provides greater stability and reduces the risk of implant migration. Preoperative assessment and careful selection of eligible patients may help reduce the risk of complications and achieve more satisfactory results. Some authors suggest routine preoperative CT scans and specific radiological protocols (Brenner et al., 2007).
The aim of this study was to describe the complications of percutaneous osseointegrated devices implanted at our hospital.
Methods
We conducted a retrospective longitudinal study in 57 consecutive patients who received a Baha® Connect percutaneous bone conduction implantation device at a tertiary hospital between January 2015 and December 2021. The external processor was selected according to bone conduction threshold hearing level, and ranged between 45-, 55-, and 65-dB HL for the Baha® 5, Baha® 5 Power and Baha® 5 SuperPower processors, respectively. All evaluated patients underwent a complete audiological evaluation including otomicroscopy, pure-tone audiometry, and speech audiometry. They also underwent preoperative testing in the clinic using an adapted device with an elastic testband for an average of two hours.
The surgical technique used was a U-shaped incision with the implant placed outside the incision. After performing the incision and dissecting the subcutaneous cellular tissue, the periosteum was elevated, a countersink created in the bone bed and the baseplate was placed in situ; the baseplate size was chosen according to the thickness of the skin and subcutaneous cellular tissue. The baseplate was exteriorised outside the incision by punch technique. The incision was sutured, and a healing cap was then attached and covered with antibiotic ointment-impregnated gauze with bacitracin, neomycin and polymyxin B.
All surgery were performed under general anaesthesia combined with infiltration of a local anaesthetic with a vasoconstrictor agent. Complications were graded according to the classification described by Holgers classification, which stratifies peri-implant skin complications (Table 1; Ellsperman et al., 2021).
Table 1. Holgers classification
Grade |
Skin reaction |
Treatment |
0 |
None |
Remove debris |
1 |
Redness |
Local treatment |
2 |
Redness + moistness |
Local treatment |
3 |
Redness + moistness + granulation tissue |
Revision surgery |
4 |
Severe reactions |
Removal |
Results
The initial sample consisted of 63 patients, of whom a total of six were excluded (three were lost to follow-up and three still had a Softband-adapted Baha® device). The final sample had 57 patients with a mean age of 49.80 ± 20.02 years, 56.89% of whom were female. The mean age of the adult subgroup was 55.26 ± 15.80 years. Of the total, seven patients (12.28%) were younger than 15 years and had a mean age of 10.42 ± 3.30 years. The mean follow-up was 12 months (range 4-48 months) and consisted of regular visits to the hearing clinic once weekly for the first two weeks, then monthly until six months and then every six months for most patients, although the schedule varied according to each patient’s progress. The Holgers classification was recorded at each visit if the patient was grade 1-4. The sound processor was programmed at the clinic at the postoperative month 1 visit.
About 80% of local peri-implant complications occurred in the first two months after surgery. However, three adult patients without medical histories of note had recurrent infections during the first years after surgery. The average follow-up period varies widely in the literature, ranging from three to 61 months in the systematic review by Mohamad et al. (2016).
Table 2 shows the clinical indications for device implantation. Tables 3 and 4 specify the clinical indications for the paediatric and adult population subgroups, respectively, in the sample. Patients with chronic otitis media reported frequent episodes of otorrhoea that either prevented the use of conventional hearing aids or limited their performance. No patients who received an implant in the sample were using a CROS (Contralateral Routing of Signals) device for unilateral profound sensorineural hearing loss.
Table 2. Clinical indications for percutaneous bone conduction device placement in the study sample
Diagnosis |
n = 57 |
Chronic simple/cholesteatomatous otitis media |
42 |
Congenital malformations of the pinna and external auditory canal |
10 |
Otosclerosis with poor response to surgery |
4 |
Poor adaptation to hearing aid |
1 |
Table 3. Clinical indications for percutaneous bone conduction device placement in the paediatric subgroup
Diagnosis |
n = 7 |
Congenital malformations of the pinna and external auditory canal |
6 |
Chronic cholesteatomatous otitis media |
1 |
Table 4. Clinical indications for percutaneous bone conduction device placement in the adult subgroup
Diagnosis |
n = 50 |
Chronic simple/cholesteatomatous otitis media |
41 |
Congenital malformations of the pinna and external auditory canal |
4 |
Otosclerosis with poor response to surgery |
4 |
Poor adaptation to hearing aid |
1 |
Of the total patients studied, 15 (26.31%) had some type of local complication. Most of these complications (93.33%) were minor (Holgers grades 1 and 2; Table 5). Complications in the paediatric subgroup were notably higher. Of the seven children who received implants, three (42.85%) had some type of complication. Two were classified as Holgers grade 1 and the third, who had tissue overgrowth on the implant requiring replacement, as Holgers grade 4 (Figure 2). One paediatric patient had spontaneous implant extrusion and another had extrusion following local trauma. In the adult subgroup, the complication rate was 24%, and all complications were classified as minor (Holgers 1 and 2). There were no major local complications graded as Holgers 3 or 4 (Figure 3).
Table 5. Peri-implant soft tissue complications categorised by Holgers grade, entire study population
Skin complications |
Treatment |
Number of patients (%) |
Holgers 0 |
None needed |
42 (73.68%) |
Holgers 1 |
None needed |
3 (5.26%) |
Holgers 2 |
Topical antibiotic + weekly revisions |
11 (19.29%) |
Holgers 3 |
Revision surgery |
0 (0.00%) |
Holgers 4 |
Implant removal |
1 (1.75%) |
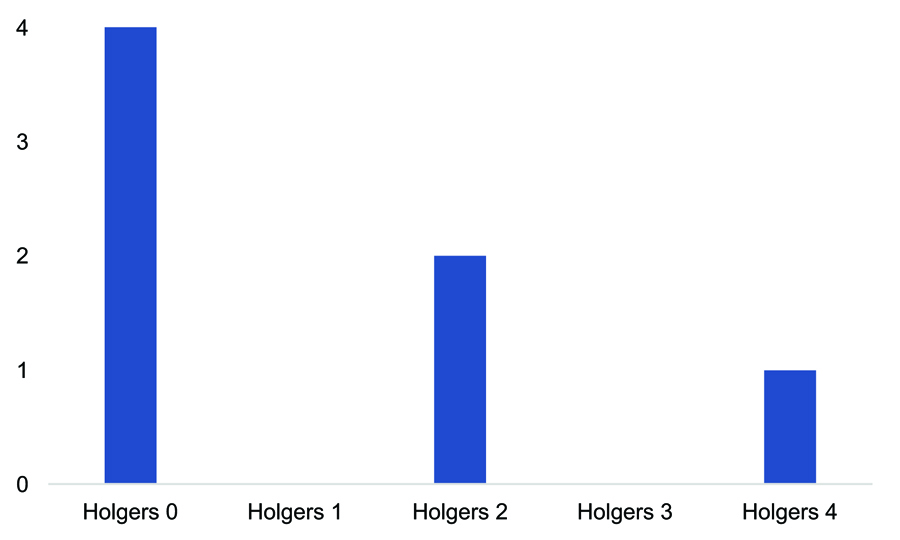
Figure 2. Skin complications in the paediatric subgroup
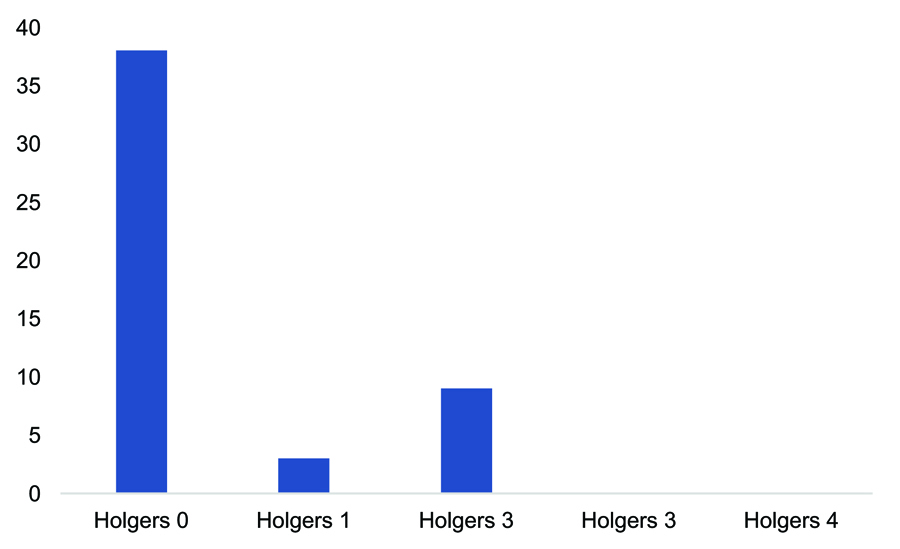
Figure 3. Skin complications in the adult subgroup
Of the patients with local complications, two were diagnosed with irritative eczema and one with psoriasis, with no other skin diseases. According to the CT studies, all patients complied with the 2.5-mm minimum thickness required for baseplate placement and therefore it was not considered a determinant for the occurrence of local complications in our sample (Tjellström et al., 2001).
Discussion
Our study had a low rate of local complications (26.31%) from percutaneous bone conduction implantation, and most complications (93.33%) were minor. However, the rate increased to 42% in the paediatric subgroup. A systematic review of Ponto® percutaneous bone conduction devices, published by Lagerkvist et al. (2020), described a local adverse reaction rate of Holgers ≥2 in 15% (133/863) of patients, although not all studies reported this information. The same systematic review found no life-threatening complications, coinciding with the data obtained in a systematic review by Schwab et al. (2020), covering a total of 234 articles, in which the incidence of adverse events was described using the ratio of events-to-ears (REE) parameter and analysed the REE separately for five categories (device-related, skin-related, surgery-related, patient-related and not specified). A total of 204 different types of adverse events were found. For Baha® Connect devices, the most frequent adverse events were Holgers grade 1 (REE 0.07), Holgers grade 2 (REE 0.05) and skin revision surgery due to overgrowth (REE 0.04). The data analysis showed that the complication rate was very low in both devices and that adverse events were not comparable because they were qualitatively different. The results reported in the systematic review corroborate the findings in our study, in which the most frequent complications were Holgers grades 1 and 2 (82.35%).
A meta-analysis by Kiringoda and Lustig (2013) found that the most common complications reported were local skin complications. After reviewing 21 articles, the complication rate in adult and mixed populations was found to range from 2.4% to 38.1% for Holgers grades 2-4 and from 0.4% to 4.8% specifically for Holgers grade <4 complications. A single study in paediatric patients reported an overall complication rate of 89% and Holgers ≥2 in 77.8% of 27 patients (Kraai et al., 2011). Other studies report implant extrusion rates in the paediatric population ranging from 5.3% to 26% (Dun et al., 2012). Roman et al. (2011) found that the most frequent complications in the paediatric age group were fixture loss and the development of skin complications, with fixture loss occurring in 14% of the overall study population and in 71% in the group of children under 5 years of age. These results are consistent with a retrospective study published by de Wolf et al. (2008) that found implant loss in 16.3% (21/129) of the paediatric population, mostly occurring during the first year after surgery.
In studies by den Besten et al. (2015) and Dun et al. (2012), skin diseases and profound learning disabilities were found to be risk factors for the development of skin complications; in contrast, female gender was considered to be a negative or protective risk factor. We did not study risk factors in our sample. Siau et al. (2016) described the main reasons for patients refusing Baha® implantation. Of 98 patients who were found suitable for this procedure, only 38.8% underwent surgery. Anxiety over surgery was reported by 45% and cosmetic concerns by 30%. Cosmetic concerns are one of the main reasons why transcutaneous devices are popular, since they maintain skin surface integrity. However, these devices are not free of complications (de Souza et al., 2022).
Although passive transcutaneous devices result in a lower rate of skin complications, the magnetic force required to hold the external device in place and ensure good sound transmission can cause pain and local irritation, leading to ischaemic necrosis in the worst cases (Chen et al., 2017; Ellsperman et al., 2021). A systematic review by Cooper et al. reported a 13.1% rate for minor skin complications, which resolved with the use of a weaker magnet (Cooper et al., 2017; Ellsperman et al., 2021). The systematic review published by de Souza et al., (2022) described a total of 192 adverse events related to 901 transcutaneous device implantations (21.3%), 84.3% of which were classified as minor and 16.1% as major. The rate of minor complications for transcutaneous implantation is around 20%, which is similar to the complication rate for percutaneous implantation.
The aim of the tissue-preservation U-shaped incision technique with external implant placement that we used in our study is to avoid complications such as bacterial overgrowth, which can lead to local complications. A retrospective study published by Strijbos et al. (2022) compared the linear incision technique with tissue preservation (LITT-P) with the minimally invasive star-shaped incision (SSI). No intraoperative complications were reported, but the rate of skin complications was lower with the LITT-P technique (17 vs. 21 for Holgers grade 1, and 10 vs. 16 Holgers grade 2). These results are consistent with the systematic review by Mohamad et al. (2016) of 30 studies that compared complication rates by surgical technique used. Fewer complications were reported with the linear incision than with the dermatome technique (Mohamad et al., 2016; Tamarit Conejeros et al., 2009). In our study, we used the same surgical technique in all patients, so we were unable to compare complication rates.
The cost of bone conduction devices varies depending on the specific model, device complexity, processor type and accessories included (Olsen et al., 2011). Cost does not influence the onset of skin complications, but we believe that this aspect should be taken into account when selecting a device, especially in public health care institutions. Complication rates vary by surgical technique, surgeon experience and patient predisposing factors (den Besten et al., 2015; Ellsperman et al., 2021; Kiringoda and Lustig, 2013). The percentage of major complications is low; and therefore, changing device-type indications due to the risk of complications is unwarranted (Ellsperman et al., 2021). Tissue-preservation techniques, such as the one used in our study, are safe, with a low incidence of postoperative infection in short- and long-term follow-ups (Verheij et al., 2016).
Limitations of our study include a heterogeneous sample population, small size and lack of a risk factor analysis. Comparing different surgical techniques (minimally invasive ponto surgery [MIPS] versus skin flap incisions) and analysing complication rates by bone conduction device both require broader studies with larger study populations. We are; therefore, considering designing a multicentre study.
Conclusions
The choice of osseointegrated device should depend on its amplification capability, cost, and complications reported at the health care institution. The rate of major complications is very low. Changing from percutaneous to transcutaneous device implantations solely to avoid local complications is unjustified.
Acknowledgements
The authors are grateful to Dr. José Luis Castillo for his advice on methodological aspects.
References
Brånemark, R., Brånemark, P. I., Rydevik, B., & Myers, R. R. (2001). Osseointegration in skeletal reconstruction and rehabilitation: a review. J Rehabil Res Dev., 38(2), 175–181.
Brenner, M. J., Goebel, J. A., & Wippold, F. J., 2nd (2007). Insufficient cranial thickness in bone-anchored hearing aid placement. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 28(6), 865–866. https://doi.org/10.1097/MAO.0b013e318030b042
Calon, T. G. A., Johansson, M. L., de Bruijn, A. J. G., van den Berge, H., Wagenaar, M., Eichhorn, E., Janssen, M. M. L., Hof, J. R., Brunings, J. W., Joore, M. A., Jonhede, S., van Tongeren, J., Holmberg, M., & Stokroos, R. J. (2018). Minimally invasive ponto surgery versus the linear incision technique with soft tissue preservation for bone conduction hearing implants: A multicenter randomized controlled trial. Otol Neurotol., 39(7), 882–893. https://doi.org/10.1097/MAO.0000000000001852
Cass, S. P., & Mudd, P. A. (2010). Bone-anchored hearing devices: indications, outcomes, and the linear surgical technique. Oper Tech Otolaryngol Head Neck Surg;21:197–206. https://doi.org/10.1016/j.otot.2010.05.004
Chen, S. Y., Mancuso, D., & Lalwani, A. K. (2017). Skin necrosis after implantation with the BAHA attract: A case report and review of the literature. Otol Neurotol., 38(3), 364–367. https://doi.org/10.1097/MAO.0000000000001327
Cooper, T., McDonald, B., & Ho, A. (2017). Passive transcutaneous bone conduction hearing implants: A systematic review. Otol Neurotol., 38(9), 1225–1232. https://doi.org/10.1097/MAO.0000000000001518
den Besten, C. A., Bosman, A. J., Nelissen, R. C., Mylanus, E. A., & Hol, M. K. (2016). Controlled clinical trial on bone-anchored hearing implants and a surgical technique with soft-tissue preservation. Otol Neurotol., 37(5), 504–512. https://doi.org/10.1097/MAO.0000000000000994
den Besten, C. A., Nelissen, R. C., Peer, P. G., Faber, H. T., Dun, C. A., de Wolf, M. J., Kunst, H. P., Cremers, C. W., Mylanus, E. A., & Hol, M. K. (2015). A retrospective cohort study on the influence of comorbidity on soft tissue reactions, revision surgery, and implant loss in bone-anchored hearing implants. Otol Neurotol., 36(5), 812–818. https://doi.org/10.1097/MAO.0000000000000745
de Souza, M. A., Vallejos Riart, S. L., Souza, S. R., de Brito, R., & Bento, R. F. (2022). Complications of transcutaneous protheses - A systematic review of publications over the past 10 years. Int Arch Otorhinolaryngol., 26(3), e505–e512. https://doi.org/10.1055/s-0042-1742352
de Wolf, M. J., Hol, M. K., Huygen, P. L., Mylanus, E. A., & Cremers, C. W. (2008). Nijmegen results with application of a bone-anchored hearing aid in children: simplified surgical technique. Ann Otol, Rhinol Laryngol., 117(11), 805–814. https://doi.org/10.1177/000348940811701103
Dufour-Fournier, C., Devèze, A., Barbut, J., Ogam, E., Saliba, I., & Masson, C. (2022). Analysis of the acoustic transcranial bone conduction. Audiol Res., 12(2), 162–170. https://doi.org/10.3390/audiolres12020019
Dun, C. A., Faber, H. T., de Wolf, M. J., Mylanus, E. A., Cremers, C. W., & Hol, M. K. (2012). Assessment of more than 1,000 implanted percutaneous bone conduction devices: skin reactions and implant survival. Otol Neurotol., 33(2), 192–198. https://doi.org/10.1097/MAO.0b013e318241c0bf
Ellsperman, S. E., Nairn, E. M., & Stucken, E. Z. (2021). Review of bone conduction hearing devices. Audiol Res., 11(2), 207–219. https://doi.org/10.3390/audiolres11020019.
Johansson, M. L., Stokroos, R. J., Banga, R., Hol, M. K., Mylanus, E. A., Savage Jones, H., Tysome, J. R., Vannucchi, P., Hof, J. R., Brunings, J. W., van Tongeren, J., Lutgert, R. W., Banerjee, A., Windfuhr, J. P., Caruso, A., Giannuzzi, A. L., Bordin, S., Hanif, J., Schart-Morén, N., … Hultcrantz, M. (2017). Short-term results from seventy-six patients receiving a bone-anchored hearing implant installed with a novel minimally invasive surgery technique. Clin Otolaryngol., 42(5), 1043–1048. https://doi.org/10.1111/coa.12803
Kiringoda, R., & Lustig, L. R. (2013). A meta-analysis of the complications associated with osseointegrated hearing aids. Otol Neurotol., 34(5), 790–794. https://doi.org/10.1097/MAO.0b013e318291c651.
Kraai, T., Brown, C., Neeff, M., & Fisher, K. (2011). Complications of bone-anchored hearing aids in pediatric patients. Int J Pediatr Otorhinolaryngol., 75(6), 749–753. https://doi.org/10.1016/j.ijporl.2011.01.018
Lagerkvist, H., Carvalho, K., Holmberg, M., Petersson, U., Cremers, C., & Hultcrantz, M. (2020). Ten years of experience with the Ponto bone-anchored hearing system: A systematic literature review. Clin Otolaryngol., 45(5), 667–680. https://doi.org/10.1111/coa.13556
Lavilla Martín de Valmaseda, M. J., Cavalle Garrido, L., Huarte Irujo, A., Núñez Batalla, F., Manrique Rodriguez, M., Ramos Macías, Á., de Paula Vernetta, C., Gil-Carcedo Sañudo, E., Lassaletta, L., Sánchez-Cuadrado, I., Espinosa Sánchez, J. M., Batuecas Caletrio, Á., & Cenjor Español, C. (2019). Clinical guideline on bone conduction implants. Guía clínica sobre implantes de conducción de vía ósea. Acta Otorrinolaringol Esp (Engl Ed)., 70(2), 105–111. https://doi.org/10.1016/j.otorri.2017.12.001
Mohamad, S., Khan, I., Hey, S. Y., & Hussain, S. S. (2016). A systematic review on skin complications of bone-anchored hearing aids in relation to surgical techniques. Eur Archs Otorhinolaryngol., 273(3), 559–565. https://doi.org/10.1007/s00405-014-3436-1
Olsen, S. Ø., Glad, H., & Nielsen, L. H. (2011). Comparison between two bone-anchored hearing instruments: BP100 and Ponto Pro. Int. J. Audiol, 28(8), 1107–1112. https://doi.org/10.3109/14992027.2011.605806
Reinfeldt, S., Håkansson, B., Taghavi, H., & Eeg-Olofsson, M. (2015). New developments in bone-conduction hearing implants: a review. Medical devices (Auckland, N.Z.), 8, 79–93. https://doi.org/10.2147/MDER.S39691
Roman, S., Nicollas, R., & Triglia, J. M. (2011). Practice guidelines for bone-anchored hearing aids in children. Eur Ann Otorhinolaryngol Head Neck Dis., 128(5), 253–258. https://doi.org/10.1016/j.anorl.2011.04.005
Schwab, B., Wimmer, W., Severens, J. L., & Caversaccio, M. D. (2020). Adverse events associated with bone-conduction and middle-ear implants: A systematic review. Eur Archs Otorhinolaryngol., 277(2), 423–438. https://doi.org/10.1007/s00405-019-05727-8
Siau, R. T., Dhillon, B., Siau, D., & Green, K. M. (2016). Bone-anchored hearing aids in conductive and mixed hearing losses: why do patients reject them?. Eur Archs Otorhinolaryngol., 273(10), 3117–3122. https://doi.org/10.1007/s00405-016-3941-5
Strijbos, R. M., Salameh, S., Bezdjian, A., Daniel, S. J., & Thomeer, H. G. (2022). The minimally invasive star-shaped incision technique and the linear incision technique with tissue preservation for percutaneous bone conduction devices: A Retrospective cohort study. Front Surg., 9, 863997. https://doi.org/10.3389/fsurg.2022.863997
Tamarit Conejeros, J. M., Dalmau Galofre, J., Murcia Puchades, V., Pons Rocher, F., Fernández Martínez, S., & Estrems Navas, P. (2009). Comparison of skin complications between dermatome and U-graft technique in BAHA surgery. Acta Otorrinolaringol Esp (Engl Ed)., 60(6), 422–427. https://doi.org/10.1016/j.otorri.2009.06.005
Tjellström, A., Håkansson, B., & Granström, G. (2001). Bone-anchored hearing aids: current status in adults and children. Otolaryngologic clinics of North America, 34(2), 337–364. https://doi.org/10.1016/s0030-6665(05)70335-2
Tjellström, A., Lindström, J., Hallén, O., Albrektsson, T., & Brånemark, P. I. (1981). Osseointegrated titanium implants in the temporal bone. A clinical study on bone-anchored hearing aids. Am J Otol., 2(4), 304–310.
Verheij, E., Bezdjian, A., Grolman, W., & Thomeer, H. G. (2016). A Systematic Review on Complications of Tissue Preservation Surgical Techniques in Percutaneous Bone Conduction Hearing Devices. Otol Neurotol., 37(7), 829–837. https://doi.org/10.1097/MAO.0000000000001091
Verstraeten, N., Zarowski, A. J., Somers, T., Riff, D., & Offeciers, E. F. (2009). Comparison of the audiologic results obtained with the bone-anchored hearing aid attached to the headband, the testband, and to the "snap" abutment. Otol Neurotol., 30(1), 70–75. https://doi.org/10.1097/MAO.0b013e31818be97a
Yuen, H. W., Bodmer, D., Smilsky, K., Nedzelski, J. M., & Chen, J. M. (2009). Management of single-sided deafness with the bone-anchored hearing aid. Otolaryngol Head Neck Surg., 141(1), 16–23. https://doi.org/10.1016/j.otohns.2009.02.029
Zernotti, M. E., & Sarasty, A. B. (2015). Active bone conduction prosthesis: Bonebridge(TM). Arch Otorhinolaryngol., 19(4), 343–348. https://doi.org/10.1055/s-0035-1564329
Conflict of interest
The authors declare no conflict of interest.
Ethical approval and informed consent
This study was conducted in accordance with the principles of the Declaration of Helsinki. All patients enrolled in the study signed the relevant informed consent form.
Author contributions
MNG: Data acquisition. Research. Data analysis and interpretation. Writing of the original draft. JCJ: Data acquisition. Data analysis and interpretation. Research. FFN: Data acquisition. Material resources. Research. Supervision. JME: Writing (review and editing). Validation. JGV: Conceptualization. Research. Data analysis and interpretation.
How to cite
Núñez-Gutiérrez, M., Castro-Jiménez, J., Fernández-Nogueras Jiménez, F., Espinosa-Sánchez, J.M., García-Valdecasas, J. (2023).
Do complications of percutaneous osseointegration justify a switch to transcutaneous devices? A retrospective longitudinal study on complications. Auditio, 7, e89.
https://doi.org/10.51445/sja.auditio.vol7.2023.0089
Correspondence
Marta Núñez Gutiérrez
Secretaría ORL, Hospital Universitario Virgen de las Nieves (HUVN), Av. de las Fuerzas Armadas, 2, 18014, Granada. España.
Email: martangutierrez@hotmail.es
Editorial Office
Copyeditor: Tomás Pérez Pazos
Translation: Emma Goldsmith
Trans. Revision: Carlos R. Benítez-Barrera
Production: Glaux Publicaciones Académicas