 1,2,3, Jose M. Bermúdez-Muñoz
1,2,3, Jose M. Bermúdez-Muñoz 1,2, Carmen Ruiz-García
1,2, Carmen Ruiz-García 4, Luis Lassaletta2,4,5, Julio Contreras
4, Luis Lassaletta2,4,5, Julio Contreras 1,2,6, Silvia Murillo-Cuesta
1,2,6, Silvia Murillo-Cuesta 1,2,5* & Isabel Varela-Nieto
1,2,5* & Isabel Varela-Nieto 1,2,5
1,2,5Research Articles
Genetic, molecular and biochemical basis of auditory ageing: lessons from experimental models
Blanca Cervantes 1,2,3, Jose M. Bermúdez-Muñoz
1,2,3, Jose M. Bermúdez-Muñoz 1,2, Carmen Ruiz-García
1,2, Carmen Ruiz-García 4, Luis Lassaletta2,4,5, Julio Contreras
4, Luis Lassaletta2,4,5, Julio Contreras 1,2,6, Silvia Murillo-Cuesta
1,2,6, Silvia Murillo-Cuesta 1,2,5* & Isabel Varela-Nieto
1,2,5* & Isabel Varela-Nieto 1,2,5
1,2,5
1Instituto de Investigaciones Biomédicas Alberto Sols CSIC-UAM, Madrid, Spain / 2Centro de Investigación Biomédica en Red, Instituto de Salud Carlos III, Madrid, Spain / 3Facultad de Medicina, Universidad Anáhuac, Puebla, Mexico / 4Servicio ORL, Hospital Universitario La Paz, Madrid, Spain / 5Instituto de Investigación Hospital Universitario La Paz, Madrid, Spain / 6Facultad de Veterinaria, Universidad Complutense de Madrid, Madrid, Spain
 OPEN ACCESS
OPEN ACCESS
PEER REVIEWED
PERSPECTIVES
Abstract
Age-related hearing loss (ARHL) affects one in three people older than 65 years and is the most prevalent sensorineural deficit in this age group. This type of hearing loss precedes and accelerates the onset of cognitive impairment and is associated with an increased risk for neurodegenerative diseases such as dementia and Alzheimer disease. The onset and progression of ARHL is influenced by genetic factors, which are still poorly understood, and environmental factors, which in particular include exposure to excessive noise and ototoxic substances. At present, no effective drug treatments are available for ARHL prevention or treatment, and therefore research in this field is a priority. In the research field, animal models offer a crucial tool for i) identifying new genes associated with ARHL, ii) understanding the cellular and molecular basis of auditory ageing and iii) defining new therapeutic targets and evaluating candidate treatments.
Keywords
ARHL; apoptosis; oxidative stress; neuroinflammation; animal models.
Clinical implications
ARHL is a complex multifactorial disorder that affects a part of the population – people older than 65 – that will increase exponentially in developed countries in the coming decades. The disorder seriously impairs older adults’ ability to communicate and adversely effects their quality of life. Furthermore, a clear association has been described with the onset of neurodegenerative diseases such as senile dementia and Alzheimer disease. Current treatment (hearing aids and cochlear implants) is expensive and inaccessible for some patients, while preventive and curative drug treatments are still at a preclinical or clinical research phase. As a result, ARHL, like other geriatric diseases, is a major challenge for the biomedical community and the healthcare system.
Received: 10.04.2022 Revised: 04.06.2022 Accepted: 06.07.2022 Published: 01.09.2022
Editor:
Gerard Encina-Llamas,
Technical University of Denmark, Denmark
Reviers:
Jordi Llorens,
University of Barcelona, Spain.
Ignacio del Castillo Fernández del Pino,
University Hospital Ramón y Cajal, Spain.
Patricia Vázquez,
Fundación Vinjoy, Spain.
Introduction
According to World Health Organization (WHO) data, about 20% of the global population has some form of hearing loss, and 6.1% – some 466 million people – have disabling hearing loss (WHO, 2021). The overall cost of unaddressed hearing loss is calculated at USD 980 billion per year, representing the sum of healthcare costs (excluding hearing assistive devices), educational support, lost productivity and social costs. Two particular causes for concern are noise-induced hearing loss (with more than one billion young people aged 12-35 estimated to be at risk for hearing loss due to noise exposure in recreational settings) and age-related hearing loss (ARHL), due to the ageing world population (WHO, 2021).
An individual is considered to have hearing loss if they have a pure-tone average loss greater than 25 dB in the better-hearing ear (Olusanya, Davis & Hoffman, 2019). Hearing loss can be mild, moderate, severe or profound and can affect one or both ears. The main clinical forms include i) congenital or early-onset hearing loss caused by genetic defects, infections during pregnancy or complications at birth; ii) hearing loss caused by chronic otitis media; iii) noise- and ototoxic medicine-induced hearing loss that damage the inner ear; and iv) age-related hearing loss.
The diverse consequences of hearing loss depend on severity and time of onset, and can be potentially disabling. Hearing loss in children prevents or delays language development and reduces school performance, while in adults it hinders social and professional development. Older adults with hearing loss experience an impaired ability to communicate with others, leading to social isolation, loneliness and depression. Furthermore, hearing loss is associated with increased cognitive frailty and faster progression of neurodegenerative diseases (Kim, Lim, Kong & Choi, 2018). The COVID-19 pandemic and other exceptional situations have further obstructed communication for people with hearing loss, because face masks prevent lip-reading, as reported in Spain by FIAPAS (Spanish Confederation of Deaf People’s Families) and CNSE (State Confederation of Deaf People).
Discussion
In humans, ageing is a physiological process characterized by the progressive and permanent accumulation of a wide variety of molecular and cellular damage, leading to generalized and progressive deterioration of many body functions and increased susceptibility to disease. The main physiopathological mechanisms related to ageing are genomic instability, epigenetic alterations, mitochondrial dysfunction, cellular senescence and loss of proteostasis (López-Otín et al., 2013). The morphological and functional changes associated with ageing have an impact on the cardiovascular, renal, nervous and muscular systems, among others. With age, structural and functional changes also occur in the ear, leading to ARHL.
Age-related hearing loss, or presbycusis, affects one-third of the population older than 65 years and half of all individuals older than 75 (WHO, 2021). With increasing life expectancy and the global ageing of the population, ARHL will be a major challenge for healthcare systems in the coming decades.
The onset of ARHL manifests as a loss of sensitivity to sound, initially at high frequencies and then progressing to lower frequencies, which hinders the ability to follow a conversation, especially in noisy environments. This situation significantly reduces older adults’ ability to communicate, their quality of life and independence. These individuals tend to withdraw themselves socially and receive less stimulation, which, together with an ageing brain, favours cognitive deterioration and the onset of neurodegenerative diseases such as senile dementia and Alzheimer disease. Cognitive impairment is estimated to be 40% higher in individuals with poor hearing than in those with good hearing (Panza, Solfrizzi & Logroscino, 2015).
An effective solution to treat ARHL and reduce cognitive frailty is the use of hearing aids, which increase the intensity of sounds received, and cochlear implants, which directly stimulate the nerve endings in the inner ear. Users of hearing aids and cochlear implants have considerably reduced auditory memory loss, improved social interaction and, in short, decreased age-related cognitive decline (Calvino et al., 2022; Maharani et al., 2018).
The aetiology of ARHL involves multiple environmental, genetic and epigenetic factors (Wang & Puel, 2020). Of the environmental factors, noise contributes most to the acceleration of ARHL. Chronic exposure to occupational or recreational noise causes mechanical damage – especially to hair cells – and metabolic disturbances from hypoxia due to vasoconstriction of the stria vascularis capillaries (Liberman, 2017). Another extrinsic factor is prolonged exposure to pharmacotherapy with potentially ototoxic drugs, including aminoglycoside antibiotics, platinum-derived antitumour drugs, diuretics such as furosemide, and some anti-inflammatory drugs such as aspirin (Joo et al., 2020). ARHL has also been associated with the presence of other common chronic diseases in older adults, such as diabetes, cardiovascular diseases, stroke and cancer (Besser, Stropahl, Urry & Launer, 2018). Lifestyle factors (diet, smoking, etc.) also contribute to the onset of ARHL, altering its clinical form and increasing its clinical heterogeneity. For example, nutritional deficiencies during ageing accelerate ARHL, although it is unclear whether the latter is a symptom, a trigger, or both (Partearroyo, Vallecillo, Pajares, Varela-Moreiras & Varela-Nieto, 2017).
In addition to the extrinsic factors involved, genetics plays a major role in hearing loss. The genetic component is yet to be fully elucidated, but contributes by making the sensorineural cells of the auditory organ more sensitive to damage. Genetic factors best explain the variability between individuals whose lifestyles are similar, and it is estimated that genetic factors could account for 40-70% of the onset of ARHL, according to twin research (Momi, Wolber, Fabiane, MacGregor & Williams, 2015). Epigenetic factors such as changes in DNA methylation and histone proteins are of emerging relevance in age-related conditions such as ARHL (Kuo, Moore, Lin & Ferrucci, 2021).
Identifying the genes that determine ARHL susceptibility and progression is key to making an early diagnosis and starting preventive treatment to reduce irreversible damage to hearing structures. However, despite numerous genome-wide association studies in patient cohorts and biobank samples, as well as in silico and in vitro analyses and animal model studies, only a few genes have been identified (Ivarsdottir et al., 2021) (Table 1).
Table 1. Main identified and candidate genes for susceptibility to age-related hearing loss in Homo sapiens.
Gene |
Function |
Reference |
Population |
ACVR1B |
Activin receptor type 1B |
Europe |
|
APOE |
Lipid transport |
USA |
|
CAT |
Catalase, antioxidant enzyme |
Europe |
|
CCBE1 |
Participates in lymphangiogenesis in vertebrates |
Europe |
|
CDH13 |
Cell adhesion |
Europe |
|
CEP104 |
Ciliogenesis and ciliary integrity |
Europe (Italy) |
|
CMIP |
T-cell signalling |
Europe |
|
DLCK1 |
Serine/threonine-protein kinase, signalling |
Europe (Italy and Croatia) |
|
EDN1 |
Vasoactive peptide expressed in ganglion cells |
Japan |
|
ESRRG |
Steroid hormone nuclear receptor |
Europe |
|
EYA4 |
Transcriptional activator, maturation of the organ of Corti |
Europe |
|
FXYD5 |
Sodium channel activity regulator |
Europe and USA |
|
GHR |
Growth hormone receptor |
(Attias, Zarchi, Nageris & Laron, 2012; Prado-Barreto et al., 2014) |
Patients with Laron syndrome from Israel; Brazil |
GRLH2 |
Epithelial development |
Europe and China |
|
GRM7 |
Synaptic transmission |
Europe and USA |
|
GRM8 |
Synaptic transmission |
Europe |
|
GSTM1 |
Glutathione S-transferases, Detoxification, antioxidant |
USA |
|
GSTT1 |
Glutathione S-transferases, Detoxification, antioxidant |
USA |
|
IGF1 |
Insulin growth factor family |
England |
|
ILDR1 |
Membrane multimeric receiver |
Europe |
|
IPP |
Interaction with actin |
Europe and USA |
|
IQGAP2 |
Cell adhesion, motility and morphology |
Saami population in Finland |
|
ISG20 |
Exonuclease |
Europe |
|
ITGA8 |
Cell adhesion |
Europe |
|
KCNMA1 |
Calcium channel |
Europe |
|
KCNQ1 |
Potassium channel |
Europe |
|
KCNQ4 |
Potassium channel |
USA |
|
MPZL2 |
Adhesion molecule. Epithelial cell interactions |
Europe |
|
mtDNA |
Mitochondrial DNA mutations, mtDNA 4977 |
Europe and China |
|
MTHFR |
Homocysteine metabolism, antioxidant |
(Manche, Jangala, Dudekula, Koralla & Akka, 2018; Uchida, Sugiura, Ando, Nakashima & Shimokata, 2011) |
India Japan |
MTR |
Homocysteine metabolism, antioxidant |
Japan |
|
NAT2 |
Detoxification, antioxidant |
Europe (general); Turkey |
|
PCDH15 |
Stereocilia structure |
Europe |
|
PCDH20 |
Cell adhesion |
Italy (isolated) and Silk Road |
|
PTPRD |
Protein tyrosine phosphatase receptor delta |
Europe |
|
PRKCE |
Protein kinase, related to the Nrf2 pathway |
Europe and Central Asia |
|
SIK3 |
Kinase, positive regulator of mTOR |
Europe Silk Road |
|
SLC28A3 |
Human concentrative nucleoside transporter |
Italy (isolated) and Silk Road |
|
SLC44A2 |
Choline transporter |
Europe |
|
SLC7A8 |
Neutral amino acid transporter |
Italy (isolated) |
|
SOD2 |
Superoxide dismutase 2 enzyme, antioxidant |
Europe |
|
SPIRE2 |
Intracellular vesicle transport along actin filaments |
Europe and USA |
|
SPTBN1 |
Secretion. Calcium-dependent movement of the cytoskeleton at the membrane |
Europe and USA |
|
STRN |
Calmodulin-binding protein |
Europe |
|
TFGB1 |
Immune response |
Europe and Central Asia |
|
TNF |
Immune response |
Japan |
|
TNFRSF1B |
Immune response |
Japan |
|
TRIL |
Immune response to LPS. Component of the TLR4 signalling complex |
Europe and USA |
|
TRIOBP |
Cytoskeleton organization, cell motility |
Europe |
|
UCP2 |
Mitochondrial proton transporter |
Japan |
|
WFS1 |
Transmembrane protein in the endoplasmic reticulum, cation-selective channel |
Finland |
The anatomical and functional complexity of the cochlea explains why variants in highly diverse genes are associated with an increased predisposition to ARHL. Specifically, genes related to mechanotransduction, potassium recycling, cell junctions and synaptic transmission account for a large part of identified genes. Other genes involved include those that participate in deregulated processes during ageing such as oxidative stress, inflammation and mitochondrial function, all of which have been described in other age-related diseases, including atherosclerosis, cancer, cataracts, osteoporosis, type 2 diabetes, hypertension and Alzheimer disease.
The cochlea is a spiralling membranous duct divided into three fluid-filled chambers or scalae (Figure 1). The organ of Corti is in the scala media and contains the hair cells that are responsible for transducing vibrations into nerve signals that are sent to neurons in the spiral ganglion. The lateral wall houses the stria vascularis, which is essential for maintaining endolymph ionic homeostasis.
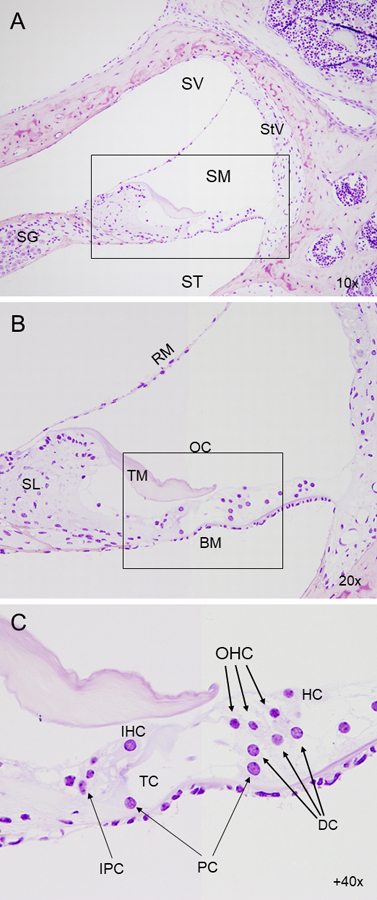
Figure 1. Cytoarchitecture of the inner ear. Histo-resin sections of mouse cochlea, with Nissl staining contrast. (A) Micrograph showing a cross-section of the cochlear duct in wild-type mouse, and the three scalae (scala vestibuli, scala media and scala tympani). (B) Detail of the scala media, containing the organ of Corti, responsible for mechanotransduction. (C) Close-up of the organ of Corti, comprised of one row of inner hair cells and three rows of outer hair cells, separated by the tunnel of Corti. The hair cells are held in place by a set of supporting cells, residing in turn on the basilar membrane. SV, scala vestibuli; SM, scala media; ST, scala tympani; SG, spiral ganglion; StV, stria vascularis; BM, basilar membrane; TM, tectorial membrane; RM, Reissner's membrane; SL, spiral ligament; OC, organ of Corti; IHC, inner hair cell; OHC, outer hair cell; IPC, inner phalangeal cell; PC, pillar cell; DC, Deiter’s cell; HC, Hensen's cell, TC, tunnel of Corti.
From a pathophysiological point of view, ARHL has traditionally been divided into three main types, depending on which structure is involved: (i) sensory ARHL, characterized by hair cell loss in the organ of Corti; (ii) strial or metabolic ARHL, caused by atrophy of the stria vascularis; and (iii) neural ARHL, caused by atrophy of the spiral ganglion and degeneration of the auditory nerve (Schuknecht & Gacek, 1993). However, most cases of ARHL are mixed, with a combination of structures involved.
Cellular mechanisms leading to ARHL are diverse and interrelated with other organs that suffer impaired functionality during ageing. These mechanisms include gene and protein instability, cellular organelle dysfunction, impaired nutrient and cellular communication processes, and loss of regenerative capacity (López-Otín, Blasco, Partridge, Serrano & Kroemer, 2013). Unlike birds and reptiles, mammals are unable to regenerate hair cells, and therefore repeated damage leads to irreparable and irreversible death. Regenerative therapies in hearing loss focus on modulating the molecular pathways that induce proliferation and transdifferentiation of supporting cells into hair cells, thus leading to their regeneration (Rai, Tu, Frank & Zuo, 2021).
With regard to the molecular mechanisms involved in ARHL, inflammation and oxidative stress have received the most attention, generating the largest number of therapeutic targets. During ageing, chronic low-grade inflammation occurs as a result of decreased immune system cell activity. Various studies have found a clear association between the increase in hearing thresholds with age and the increase in the number of activated macrophages, overexpression of inflammatory genes and proinflammatory cytokines in the cochlea (Paplou, Schubert & Pyott, 2021).
Oxidative stress results from an imbalance between the amount of reactive oxygen and nitrogen species (ROS, RNS) and the cell's antioxidant defences, mainly the enzymes from glutathione metabolism. Free radicals are physiologically produced during cellular respiration in the mitochondria, but their production is exacerbated by the effect of environmental factors such as noise or ototoxic drugs. Nutritional deficiencies and specific genetic polymorphisms may decrease antioxidant enzyme activity and contribute to oxidative stress. An excess of reactive species damages DNA, lipid and protein molecules, leading to activation of cell death by apoptosis.
There is a dearth of clinical research in hearing loss, mainly because of the inaccessible nature of the inner ear, and therefore research chiefly focuses on history taking, electrophysiological testing and, sometimes, imaging techniques. Other procedures such as biopsies, blood biomarker tests and post-mortem histology analyses are, however, rare. Experimental in silico, cellular and, mainly, animal models have been key to i) understanding auditory anatomy and physiology; ii) understanding the genetic and molecular basis of hearing loss; iii) defining therapeutic targets and evaluating drugs that may be able to delay, reduce or reverse pathological changes in auditory neurosensory cells during ageing; and iv) developing diagnostic tests such as auditory evoked potentials and otoacoustic emissions, and devices such as cochlear implants.
The most commonly used animal model in hearing research and specifically in ARHL is the laboratory mouse (Mus musculus) (Bowl & Dawson, 2015). Mice live an average of 2-2.5 years and are considered models of ageing from 18 months of age. At this age, mice show symptoms of auditory ageing similar to those of humans, both functionally – with increased thresholds at high frequencies – and morphologically – with loss of hair cells and spiral ganglion neurons (Figure 2).
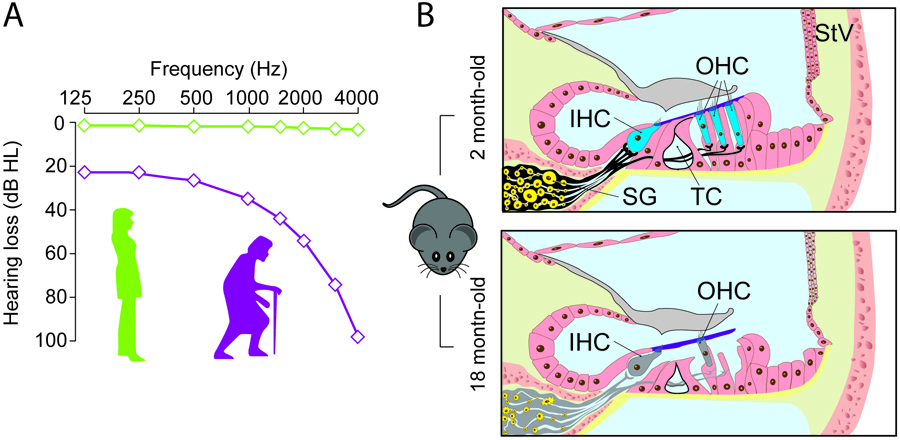
Figure 2. Use of the mouse model in ARHL studies. (A) Diagram depicting ARHL. Young people (green line) have no hearing loss at any of the frequencies studied. In ageing adults (purple line), hearing thresholds increase in response to high frequencies, progressively extending to lower frequencies. (B) Mice are excellent models for studying changes in cochlear cytoarchitecture during ageing. Compared to a young 2-month-old mouse, an 18-month-old mouse shows loss of outer hair cells (OHC), inner hair cells (IHC), spiral ganglion (SG) neurons, and synaptic connections between the two cell types. Other common changes are tunnel of Corti (TC) closure or collapse and stria vascularis (StV) atrophy. These morphological changes appear early in C57BL/6J mice, making this strain a classic model for ARHL study.
However, in some genetically modified mouse strains, such as SAMP (Senescence-accelerated mouse prone) mice, the ageing process is accelerated and the mice develop geriatric pathologies such as osteoporosis, amyloidosis, memory loss and ARHL at an early age (Marie, Larroze-Chicot, Cosnier-Pucheu & Gonzalez-Gonzalez, 2017). Certain mouse strains have been generated to develop early-onset ARHL alone (without other symptoms). One example is the C57BL/6J mouse, which shows increased hearing thresholds from 6 months of age. This strain has been used as an ARHL model since the 1980s, although it took two more decades to locate the responsible allele (Ahl1 on mouse chromosome 10) and identify the genetic cause – a polymorphism in the Cdh23 gene, which encodes a hair cell protein (Noben-Trauth, Zheng & Johnson, 2003).
The generation of genetically modified knockout mice has been instrumental in the identification of genes associated with the onset of ARHL (Bowl & Dawson, 2015). For example, our research group has identified new genes predisposed to ARHL in mice, such as Igf1 and Dusp1 (Celaya et al., 2019; Celaya et al., 2021). Insulin-like growth factor type 1 (IGF-1), a trophic factor produced by the liver, shows decreasing levels with age. A correlation has been confirmed between IGF-1 and ARHL progression in mammals (Lassale et al., 2017). Homozygous mutations of the IGF1 gene cause hearing loss in humans and mice (Cediel, Riquelme, Contreras, Díaz & Varela-Nieto, 2006), while heterozygous mutations in mice are associated with ARHL and susceptibility to noise damage (Celaya et al., 2021; Riquelme et al., 2010). In addition, early-onset hearing loss from 4 months of age is displayed in mutant mice with inactivated dual phosphatase (DUSP1), an intracellular target of IGF-1 and a key component in oxidative stress and inflammatory response regulation (Bermúdez-Muñoz et al., 2021; Celaya et al., 2019). Similarly, studies that characterize mutant Mpzl2 and Slc7a8 knockout mice models have confirmed that these genes also increase susceptibility to auditory ageing in the mouse, and have proposed the molecular mechanisms responsible for predisposition to ARHL, which would not have been possible to describe in patients (Espino-Guarch et al., 2018; Wesdorp et al., 2018).
Animal models are also useful for studying the effect of environmental factors on auditory ageing. Many studies have shown that chronic exposure to low-level noise contributes to the onset of ARHL. Indeed, some susceptibility genes for ARHL, such as Cdh23 and Igf1, are also involved in increased sensitivity to noise-induced hearing loss, (Celaya et al., 2021; Holme & Steel, 2004). Genome-wide association studies in different mouse strains have identified a number of loci that appear to be associated with increased sensitivity to noise-induced and age-related hearing loss (Lavinsky et al., 2016). Another extrinsic factor that has been investigated in mice is nutrition and its effect on ARHL onset. Studies confirm that certain nutritional deficiencies, for example, in folic acid and B vitamins, can aggravate ARHL, while omega-3 fatty acid supplementation can ameliorate ARHL in susceptible strains such as C57 mice (Martínez-Vega, Garrido, et al., 2015; Martínez-Vega, Partearroyo, et al., 2015).
Finally, preclinical research with animal models plays an essential role – and is legally required – when evaluating the safety and efficacy of new drugs to prevent or delay ARHL onset. Many drug therapies and nutritional, gene and cell therapies are currently being studied for their potential to prevent the onset and/or slow the progression of hearing loss. Excessive free radical production and depletion of cellular antioxidant systems are key mechanisms in ARHL onset, and therefore reducing oxidative stress is an interesting strategy in ARHL prevention and treatment (Pak, Kim, Yi & Chung, 2020). Several molecules with antioxidant effects, such as N-acetyl cysteine, resveratrol and lipoic acid, have been evaluated in murine models of ARHL and been found to have beneficial effects (Huang et al., 2020; Marie et al., 2018; Muderris et al., 2022). In humans, the administration of antioxidant combinations (vitamins, lipoic acid) and dietary supplements have shown effectiveness in slowing ARHL (Durga, Verhoef, Anteunis, Schouten & Kok, 2007; Polanski & Cruz, 2013; Takumida & Anniko, 2009).
Together with drug therapies, experimental gene and cell therapies may play a role in the prevention of age-related cochlear deterioration and the replacement of degenerated cells with new functional cells (Nourbakhsh et al., 2021). New gene-editing tools have opened the door to a wide range of opportunities for treating some genetic forms of congenital hearing loss that could also offer new perspectives on ARHL in the future (Botto, Dalkara & El-Amraoui, 2021).
Conclusions
Age-related hearing loss is a major concern because of the ageing global population and its direct association with cognitive decline. Recognizing the problem of ARHL and developing an active policy to reduce risk factors and expand health coverage for people with hearing loss would help keep older adults’ active and independent for longer. Of equal importance is increasing society’s awareness regarding the prevention and early detection of hearing loss. In addition, investing in research is a priority for developing treatments to prevent degenerative changes in the inner ear.
Recent years have brought about a revolution in ARHL research, led by new genetic techniques, progress in advanced cell therapies, electronics and sequencing, prospects of artificial intelligence in diagnostics, developments in medical imaging and advanced microscopy, all of which together offer a positive outlook for patients in the future. Multidisciplinary hearing research is clearly making progress. In the short and medium term this research will contribute to increasing the wide range of diagnostic tests and treatment options which, together with hearing aids and cochlear implants, will improve the quality of life of older adults and all individuals with hearing loss.
References
Attias, J., Zarchi, O., Nageris, B. I. & Laron, Z. (2012). Cochlear hearing loss in patients with Laron syndrome. Eur Arch Otorhinolaryngol, 269(2), 461–466. https://doi.org/10.1007/s00405-011-1668-x
Bai, U., Seidman, M. D., Hinojosa, R. & Quirk, W. S. (1997). Mitochondrial DNA deletions associated with aging and possibly presbycusis: A human archival temporal bone study. Am J Otol, 18(4), 449–453.
Bared, A., Ouyang, X., Angeli, S., Du, L. L., Hoang, K., Yan, D. & Liu, X. Z. (2010). Antioxidant enzymes, presbycusis, and ethnic variability. Otolaryngol Head Neck Surg, 143(2), 263–268. https://doi.org/10.1016/j.otohns.2010.03.024
Bermudez-Munoz, J. M., Celaya, A. M., Garcia-Mato, A., Munoz-Espin, D., Rodriguez-de la Rosa, L., Serrano, M. & Varela-Nieto, I. (2021). Dual-Specificity Phosphatase 1 (DUSP1) Has a Central Role in Redox Homeostasis and Inflammation in the Mouse Cochlea. Antioxidants (Basel), 10(9), 1351. https://doi.org/10.3390/antiox10091351
Besser, J., Stropahl, M., Urry, E. & Launer, S. (2018). Comorbidities of hearing loss and the implications of multimorbidity for audiological care. Hear Res, 369, 3–14. https://doi.org/10.1016/j.heares.2018.06.008
Botto, C., Dalkara, D. & El-Amraoui, A. (2021). Progress in Gene Editing Tools and Their Potential for Correcting Mutations Underlying Hearing and Vision Loss. Front Genome Ed, 3, 737632. https://doi.org/10.3389/fgeed.2021.737632
Bowl, M. R. & Dawson, S. J. (2015). The mouse as a model for age-related hearing loss—A mini-review. Gerontology, 61(2), 149–157. https://doi.org/10.1159/000368399
Calvino, M., Sanchez-Cuadrado, I., Gavilan, J., Gutierrez-Revilla, M. A., Polo, R. & Lassaletta, L. (2022). Effect of cochlear implantation on cognitive decline and quality of life in younger and older adults with severe-to-profound hearing loss. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-022-07253-6
Camarero, G., Villar, M. A., Contreras, J., Fernandez-Moreno, C., Pichel, J. G., Avendano, C. & Varela-Nieto, I. (2002). Cochlear abnormalities in insulin-like growth factor-1 mouse mutants. Hear Res, 170(1–2), 2–11. https://doi.org/10.1016/s0378-5955(02)00447-1
Cediel, R., Riquelme, R., Contreras, J., Diaz, A. & Varela-Nieto, I. (2006). Sensorineural hearing loss in insulin-like growth factor I-null mice: A new model of human deafness. Eur J Neurosci, 23(2), 587–590. https://doi.org/10.1111/j.1460-9568.2005.04584.x
Celaya, A. M., Rodriguez-de la Rosa, L., Bermudez-Munoz, J. M., Zubeldia, J. M., Roma-Mateo, C., Avendano, C., Pallardo, F. V. & Varela-Nieto, I. (2021). IGF-1 Haploinsufficiency Causes Age-Related Chronic Cochlear Inflammation and Increases Noise-Induced Hearing Loss. Cells, 10(7). https://doi.org/10.3390/cells10071686
Celaya, A. M., Sanchez-Perez, I., Bermudez-Munoz, J. M., Rodriguez-de la Rosa, L., Pintado-Berninches, L., Perona, R., Murillo-Cuesta, S. & Varela-Nieto, I. (2019). Deficit of mitogen-activated protein kinase phosphatase 1 (DUSP1) accelerates progressive hearing loss. Elife, 8, 39159. https://doi.org/10.7554/eLife.39159
Di Stazio, M., Morgan, A., Brumat, M., Bassani, S., Dell’Orco, D., Marino, V., Garagnani, P., Giuliani, C., Gasparini, P. & Girotto, G. (2020). New age-related hearing loss candidate genes in humans: An ongoing challenge. Gene, 742, 144561. https://doi.org/10.1016/j.gene.2020.144561
Durga, J., Verhoef, P., Anteunis, L. J., Schouten, E. & Kok, F. J. (2007). Effects of folic acid supplementation on hearing in older adults: A randomized, controlled trial. Ann Intern Med, 146(1), 1–9. https://doi.org/10.7326/0003-4819-146-1-200701020-00003
Espino Guarch, M., Font-Llitjós, M., Murillo-Cuesta, S., Errasti- Murugarren, E., Celaya, A. M., Girotto, G., Vuckovic, D., Mezzavilla, M., Vilches, C., Bodoy, S., Sahún, I., González, L., Prat, E., Zorzano, A., Dierssen, M., Varela-Nieto, I., Gasparini, P., Palacín, M. & Nunes, V. (2018). Mutations in L-type amino acid transporter-2 support SLC7A8 as a novel gene involved in age-related hearing loss. ELife, 7, 31511. https://doi.org/10.7554/eLife.31511.028
Fetoni, A. R., Zorzi, V., Paciello, F., Ziraldo, G., Peres, C., Raspa, M., Scavizzi, F., Salvatore, A. M., Crispino, G., Tognola, G., Gentile, G., Spampinato, A. G., Cuccaro, D., Guarnaccia, M., Morello, G., Van Camp, G., Fransen, E., Brumat, M., Girotto, G., … Mammano, F. (2018). Cx26 partial loss causes accelerated presbycusis by redox imbalance and dysregulation of Nfr2 pathway. Redox Biol, 19, 301–317. https://doi.org/10.1016/j.redox.2018.08.002
Fransen, E., Bonneux, S., Corneveaux, J. J., Schrauwen, I., Di Berardino, F., White, C. H., Ohmen, J. D., Van de Heyning, P., Ambrosetti, U., Huentelman, M. J., Van Camp, G. & Friedman, R. A. (2015). Genome-wide association analysis demonstrates the highly polygenic character of age-related hearing impairment. Eur J Hum Genet, 23(1), 110–115. https://doi.org/10.1038/ejhg.2014.56
Friedman, R. A., Van Laer, L., Huentelman, M. J., Sheth, S. S., Van Eyken, E., Corneveaux, J. J., Tembe, W. D., Halperin, R. F., Thorburn, A. Q., Thys, S., Bonneux, S., Fransen, E., Huyghe, J., Pyykko, I., Cremers, C. W., Kremer, H., Dhooge, I., Stephens, D., Orzan, E., … Van Camp, G. (2009). GRM7 variants confer susceptibility to age-related hearing impairment. Hum Mol Genet, 18(4), 785–796. https://doi.org/10.1093/hmg/ddn402
Girotto, G., Pirastu, N., Sorice, R., Biino, G., Campbell, H., d’Adamo, A. P., Hastie, N. D., Nutile, T., Polasek, O., Portas, L., Rudan, I., Ulivi, S., Zemunik, T., Wright, A. F., Ciullo, M., Hayward, C., Pirastu, M. & Gasparini, P. (2011). Hearing function and thresholds: A genome-wide association study in European isolated populations identifies new loci and pathways. J Med Genet, 48(6), 369–374. https://doi.org/10.1136/jmg.2010.088310
Hoffmann, T. J., Keats, B. J., Yoshikawa, N., Schaefer, C., Risch, N. & Lustig, L. R. (2016). A Large Genome-Wide Association Study of Age-Related Hearing Impairment Using Electronic Health Records. PLoS Genet, 12(10), e1006371 https://doi.org/10.1371/journal.pgen.1006371
Holme, R. H. & Steel, K. P. (2004). Progressive hearing loss and increased susceptibility to noise-induced hearing loss in mice carrying a Cdh23 but not a Myo7a mutation. J Assoc Res Otolaryngol, 5(1), 66–79. https://doi.org/10.1007/s10162-003-4021-2
Huang, S., Xu, A., Sun, X., Shang, W., Zhou, B., Xie, Y., Zhao, M., Li, P., Lu, P., Liu, T. & Han, F. (2020). Otoprotective Effects of alpha-lipoic Acid on A/J Mice With Age-related Hearing Loss. Otol Neurotol, 41(6), e648–e654. https://doi.org/10.1097/MAO.0000000000002643
Ivarsdottir, E. V., Holm, H., Benonisdottir, S., Olafsdottir, T., Sveinbjornsson, G., Thorleifsson, G., Eggertsson, H. P., Halldorsson, G. H., Hjorleifsson, K. E., Melsted, P., Gylfason, A., Arnadottir, G. A., Oddsson, A., Jensson, B. O., Jonasdottir, A., Jonasdottir, A., Juliusdottir, T., Stefansdottir, L., Tragante, V., … Stefansson, K. (2021). The genetic architecture of age-related hearing impairment revealed by genome-wide association analysis. Commun Biol, 4(1), 706. https://doi.org/10.1038/s42003-021-02224-9
Joo, Y., Cruickshanks, K. J., Klein, B. E. K., Klein, R., Hong, O. & Wallhagen, M. I. (2020). The Contribution of Ototoxic Medications to Hearing Loss Among Older Adults. J Gerontol A Biol Sci Med Sci, 75(3), 561–566. https://doi.org/10.1093/gerona/glz166
Kim, S. Y., Lim, J. S., Kong, I. G. & Choi, H. G. (2018). Hearing impairment and the risk of neurodegenerative dementia: A longitudinal follow-up study using a national sample cohort. Sci Rep, 8(1), 15266. https://doi.org/10.1038/s41598-018-33325-x
Kuo, P. L., Moore, A. Z., Lin, F. R. & Ferrucci, L. (2021). Epigenetic Age Acceleration and Hearing: Observations From the Baltimore Longitudinal Study of Aging. Front Aging Neurosci, 13, 790926. https://doi.org/10.3389/fnagi.2021.790926
Kytövuori, L., Hannula, S., Mäki-Torkko, E., Sorri, M. & Majamaa, K. (2017). A nonsynonymous mutation in the WFS1 gene in a Finnish family with age-related hearing impairment. Hearing Research, 355, 97–101. https://doi.org/10.1016/j.heares.2017.09.013
Lassale, C., Batty, G. D., Steptoe, A. & Zaninotto, P. (2017). Insulin-like Growth Factor 1 in relation to future hearing impairment: Findings from the English Longitudinal Study of Ageing. Sci Rep, 7(1), 4212. https://doi.org/10.1038/s41598-017-04526-7
Lavinsky, J., Ge, M., Crow, A. L., Pan, C., Wang, J., Salehi, P., Myint, A., Eskin, E., Allayee, H., Lusis, A. J. & Friedman, R. A. (2016). The Genetic Architecture of Noise-Induced Hearing Loss: Evidence for a Gene-by-Environment Interaction. G3 (Bethesda), 6(10), 3219–3228. https://doi.org/10.1534/g3.116.032516
Liberman, M. C. (2017). Noise-induced and age-related hearing loss: New perspectives and potential therapies. F1000Res, 6, 927. https://doi.org/10.12688/f1000research.11310.1
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. (2013). The hallmarks of aging. Cell, 153(6), 1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Maharani, A., Dawes, P., Nazroo, J., Tampubolon, G., Pendleton, N. & group, S. E.-C. W. (2018). Longitudinal Relationship Between Hearing Aid Use and Cognitive Function in Older Americans. J Am Geriatr Soc, 66(6), 1130–1136. https://doi.org/10.1111/jgs.15363
Manche, S. K., Jangala, M., Dudekula, D., Koralla, M. & Akka, J. (2018). Polymorphisms in folate metabolism genes are associated with susceptibility to presbycusis. Life Sci, 196, 77–83. https://doi.org/10.1016/j.lfs.2018.01.015
Marie, A., Larroze-Chicot, P., Cosnier-Pucheu, S. & Gonzalez-Gonzalez, S. (2017). Senescence-accelerated mouse prone 8 (SAMP8) as a model of age-related hearing loss. Neurosci Lett, 656, 138–143. https://doi.org/10.1016/j.neulet.2017.07.037
Marie, A., Meunier, J., Brun, E., Malmstrom, S., Baudoux, V., Flaszka, E., Naert, G., Roman, F., Cosnier-Pucheu, S. & Gonzalez-Gonzalez, S. (2018). N-acetylcysteine Treatment Reduces Age-related Hearing Loss and Memory Impairment in the Senescence-Accelerated Prone 8 (SAMP8) Mouse Model. Aging Dis, 9(4), 664–673. https://doi.org/10.14336/AD.2017.0930
Martinez-Vega, R., Garrido, F., Partearroyo, T., Cediel, R., Zeisel, S. H., Martinez-Alvarez, C., Varela-Moreiras, G., Varela-Nieto, I. & Pajares, M. A. (2015). Folic acid deficiency induces premature hearing loss through mechanisms involving cochlear oxidative stress and impairment of homocysteine metabolism. FASEB J, 29(2), 418–432. https://doi.org/10.1096/fj.14-259283
Martinez-Vega, R., Partearroyo, T., Vallecillo, N., Varela-Moreiras, G., Pajares, M. A. & Varela-Nieto, I. (2015). Long-term omega-3 fatty acid supplementation prevents expression changes in cochlear homocysteine metabolism and ameliorates progressive hearing loss in C57BL/6J mice. J Nutr Biochem, 26(12), 1424–1433. https://doi.org/10.1016/j.jnutbio.2015.07.011
Momi, S. K., Wolber, L. E., Fabiane, S. M., MacGregor, A. J. & Williams, F. M. (2015). Genetic and Environmental Factors in Age-Related Hearing Impairment. Twin Res Hum Genet, 18(4), 383–392. https://doi.org/10.1017/thg.2015.35
Muderris, T., Yar Saglam, A. S., Unsal, D., Mulazimoglu, S., Sevil, E. & Kayhan, H. (2022). Efficiency of resveratrol in the prevention and treatment of age-related hearing loss. Exp Ther Med, 23(1), 40. https://doi.org/10.3892/etm.2021.10962. PMID: 34849155
Nagtegaal, A. P., Broer, L., Zilhao, N. R., Jakobsdottir, J., Bishop, C. E., Brumat, M., Christiansen, M. W., Cocca, M., Gao, Y., Heard-Costa, N. L., Evans, D. S., Pankratz, N., Pratt, S. R., Price, T. R., Spankovich, C., Stimson, M. R., Valle, K., Vuckovic, D., Wells, H., … Goedegebure, A. (2019). Genome-wide association meta-analysis identifies five novel loci for age-related hearing impairment. Sci Rep, 9(1), 15192. https://doi.org/10.1038/s41598-019-51630-x
Newman, D. L., Fisher, L. M., Ohmen, J., Parody, R., Fong, C. T., Frisina, S. T., Mapes, F., Eddins, D. A., Robert Frisina, D., Frisina, R. D. & Friedman, R. A. (2012). GRM7 variants associated with age-related hearing loss based on auditory perception. Hear Res, 294(1–2), 125–132. https://doi.org/10.1016/j.heares.2012.08.016
Noben-Trauth, K., Zheng, Q. Y. & Johnson, K. R. (2003). Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet, 35(1), 21–23. https://doi.org/10.1038/ng1226
Nolan, L. S., Cadge, B. A., Gomez-Dorado, M. & Dawson, S. J. (2013). A functional and genetic analysis of SOD2 promoter variants and their contribution to age-related hearing loss. Mech Ageing Dev, 134(7–8), 298–306. https://doi.org/10.1016/j.mad.2013.02.009
Nolan, L. S., Maier, H., Hermans-Borgmeyer, I., Girotto, G., Ecob, R., Pirastu, N., Cadge, B. A., Hubner, C., Gasparini, P., Strachan, D. P., Davis, A. & Dawson, S. J. (2013). Estrogen-related receptor gamma and hearing function: Evidence of a role in humans and mice. Neurobiol Aging, 34(8), 2077 e1-9. https://doi.org/10.1016/j.neurobiolaging.2013.02.009
Nourbakhsh, A., Colbert, B. M., Nisenbaum, E., El-Amraoui, A., Dykxhoorn, D. M., Koehler, K. R., Chen, Z. Y. & Liu, X. Z. (2021). Stem Cells and Gene Therapy in Progressive Hearing Loss: The State of the Art. J Assoc Res Otolaryngol, 22(2), 95–105. https://doi.org/10.1007/s10162-020-00781-0
O’Grady, G., Boyles, A. L., Speer, M., DeRuyter, F., Strittmatter, W. & Worley, G. (2007). Apolipoprotein E alleles and sensorineural hearing loss. Int J Audiol, 46(4), 183–186. https://doi.org/10.1080/14992020601145294
Olusanya, B. O., Davis, A. C. & Hoffman, H. J. (2019). Hearing loss grades and the International classification of functioning, disability and health. Bull World Health Organ, 97(10), 725–728. https://doi.org/10.2471/BLT.19.230367
Pak, J. H., Kim, Y., Yi, J. & Chung, J. W. (2020). Antioxidant Therapy against Oxidative Damage of the Inner Ear: Protection and Preconditioning. Antioxidants (Basel), 9(11), 1076. https://doi.org/10.3390/antiox9111076
Panza, F., Solfrizzi, V. & Logroscino, G. (2015). Age-related hearing impairment-a risk factor and frailty marker for dementia and AD. Nat Rev Neurol, 11(3), 166–175. https://doi.org/10.1038/nrneurol.2015.12
Paplou, V., Schubert, N. M. A. & Pyott, S. J. (2021). Age-Related Changes in the Cochlea and Vestibule: Shared Patterns and Processes. Front Neurosci, 15, 680856. https://doi.org/10.3389/fnins.2021.680856
Partearroyo, T., Vallecillo, N., Pajares, M. A., Varela-Moreiras, G. & Varela-Nieto, I. (2017). Cochlear Homocysteine Metabolism at the Crossroad of Nutrition and Sensorineural Hearing Loss. Front Mol Neurosci, 10, 107. https://doi.org/10.3389/fnmol.2017.00107
Polanski, J. F. & Cruz, O. L. (2013). Evaluation of antioxidant treatment in presbyacusis: Prospective, placebo-controlled, double-blind, randomised trial. J Laryngol Otol, 127(2), 134–141. https://doi.org/10.1017/S0022215112003118
Prado-Barreto, V. M., Salvatori, R., Santos Junior, R. C., Brandao-Martins, M. B., Correa, E. A., Garcez, F. B., Valenca, E. H., Souza, A. H., Pereira, R. M., Nunes, M. A., D’Avila, J. S. & Aguiar-Oliveira, M. H. (2014). Hearing status in adult individuals with lifetime, untreated isolated growth hormone deficiency. Otolaryngol Head Neck Surg, 150(3), 464–471. https://doi.org/10.1177/0194599813517987
Rai, V., Tu, S., Frank, J. R. & Zuo, J. (2021). Molecular Pathways Modulating Sensory Hair Cell Regeneration in Adult Mammalian Cochleae: Progress and Perspectives. Int J Mol Sci, 23(1). https://doi.org/10.3390/ijms23010066
Riquelme, R., Cediel, R., Contreras, J., la Rosa Lourdes, R. D., Murillo-Cuesta, S., Hernandez-Sanchez, C., Zubeldia, J. M., Cerdan, S. & Varela-Nieto, I. (2010). A comparative study of age-related hearing loss in wild type and insulin-like growth factor I deficient mice. Front Neuroanat, 4, 27. https://doi.org/10.3389/fnana.2010.00027
Schuknecht, H. F. & Gacek, M. R. (1993). Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol, 102(1 Pt 2), 1–16. https://doi.org/10.1177/00034894931020S101
Sugiura S, Uchida Y, Nakashima T, Ando F, Shimokata H. (2010). The association between gene polymorphisms in uncoupling proteins and hearing impairment in Japanese elderly. Acta Otolaryngol., 130(4), 487-492. https://doi.org/10.3109/00016480903283758. PMID: 19895332.
Takumida, M. & Anniko, M. (2009). Radical scavengers for elderly patients with age-related hearing loss. Acta Otolaryngol, 129(1), 36–44. https://doi.org/10.1080/00016480802008215
Uchida Y, Sugiura S, Ueda H, Nakashima T, Ando F, Shimokata H. (2014). The association between hearing impairment and polymorphisms of genes encoding inflammatory mediators in Japanese aged population. Immun Ageing, 11(1), 18. https://doi.org/10.1186/s12979-014-0018-4. PMID: 25469152; PMCID: PMC4252019
Uchida, Y., Sugiura, S., Ando, F., Nakashima, T. & Shimokata, H. (2011). Hearing impairment risk and interaction of folate metabolism related gene polymorphisms in an aging study. BMC Med Genet, 12(1), 35. https://doi.org/10.1186/1471-2350-12-35
Uchida, Y., Sugiura, S., Nakashima, T., Ando, F. & Shimokata, H. (2009). Endothelin-1 gene polymorphism and hearing impairment in elderly Japanese. Laryngoscope, 119(5), 938–943. https://doi.org/10.1002/lary.20181
Unal, M., Tamer, L., Dogruer, Z. N., Yildirim, H., Vayisoglu, Y. & Camdeviren, H. (2005). N-acetyltransferase 2 gene polymorphism and presbycusis. Laryngoscope, 115(12), 2238–2241. https://doi.org/10.1097/01.mlg.0000183694.10583.12
Van Eyken, E., Van Camp, G., Fransen, E., Topsakal, V., Hendrickx, J. J., Demeester, K., Van de Heyning, P., Maki-Torkko, E., Hannula, S., Sorri, M., Jensen, M., Parving, A., Bille, M., Baur, M., Pfister, M., Bonaconsa, A., Mazzoli, M., Orzan, E., Espeso, A., … Van Laer, L. (2007). Contribution of the N-acetyltransferase 2 polymorphism NAT2*6A to age-related hearing impairment. J Med Genet, 44(9), 570–578. https://doi.org/10.1136/jmg.2007.049205
Van Eyken, E., Van Laer, L., Fransen, E., Topsakal, V., Lemkens, N., Laureys, W., Nelissen, N., Vandevelde, A., Wienker, T., Van De Heyning, P. & Van Camp, G. (2006). KCNQ4: A gene for age-related hearing impairment? Hum Mutat, 27(10), 1007–1016. https://doi.org/10.1002/humu.20375
Van Laer, L., Huyghe, J. R., Hannula, S., Van Eyken, E., Stephan, D. A., Maki-Torkko, E., Aikio, P., Fransen, E., Lysholm-Bernacchi, A., Sorri, M., Huentelman, M. J. & Van Camp, G. (2010). A genome-wide association study for age-related hearing impairment in the Saami. Eur J Hum Genet, 18(6), 685–693. https://doi.org/10.1038/ejhg.2009.234
Van Laer, L., Van Eyken, E., Fransen, E., Huyghe, J. R., Topsakal, V., Hendrickx, J. J., Hannula, S., Maki-Torkko, E., Jensen, M., Demeester, K., Baur, M., Bonaconsa, A., Mazzoli, M., Espeso, A., Verbruggen, K., Huyghe, J., Huygen, P., Kunst, S., Manninen, M., … Van Camp, G. (2008). The grainyhead like 2 gene (GRHL2), alias TFCP2L3, is associated with age-related hearing impairment. Hum Mol Genet, 17(2), 159–169. https://doi.org/10.1093/hmg/ddm292
Vuckovic, D., Dawson, S., Scheffer, D. I., Rantanen, T., Morgan, A., Di Stazio, M., Vozzi, D., Nutile, T., Concas, M. P., Biino, G., Nolan, L., Bahl, A., Loukola, A., Viljanen, A., Davis, A., Ciullo, M., Corey, D. P., Pirastu, M., Gasparini, P. & Girotto, G. (2015). Genome-wide association analysis on normal hearing function identifies PCDH20 and SLC28A3 as candidates for hearing function and loss. Hum Mol Genet, 24(19), 5655–5664. https://doi.org/10.1093/hmg/ddv279
Wang, J. & Puel, J. L. (2020). Presbycusis: An Update on Cochlear Mechanisms and Therapies. J Clin Med, 9(1), 218. https://doi.org/10.3390/jcm9010218
Wesdorp, M., Murillo-Cuesta, S., Peters, T., Celaya, A. M., Oonk, A., Schraders, M., Oostrik, J., Gomez-Rosas, E., Beynon, A. J., Hartel, B. P., Okkersen, K., Koenen, H., Weeda, J., Lelieveld, S., Voermans, N. C., Joosten, I., Hoyng, C. B., Lichtner, P., Kunst, H. P. M., … Kremer, H. (2018). MPZL2, Encoding the Epithelial Junctional Protein Myelin Protein Zero-like 2, Is Essential for Hearing in Man and Mouse. Am J Hum Genet, 103(1), 74–88. https://doi.org/10.1016/j.ajhg.2018.05.011
Wolber, L. E., Girotto, G., Buniello, A., Vuckovic, D., Pirastu, N., Lorente-Canovas, B., Rudan, I., Hayward, C., Polasek, O., Ciullo, M., Mangino, M., Steves, C., Concas, M. P., Cocca, M., Spector, T. D., Gasparini, P., Steel, K. P. & Williams, F. M. (2014). Salt-inducible kinase 3, SIK3, is a new gene associated with hearing. Hum Mol Genet, 23(23), 6407–6418. https://doi.org/10.1093/hmg/ddu346
World Health Organization. (2021). World Hearing Report (No. 9789240020481). https://www.who.int/publications/i/item/world-report-on-hearing
Conflicts of interest
The authors declare no conflict of interest.
Author contributions
BC, JMB and SM, manuscript writing and editing; CRG, LL and JC, manuscript revision; IVN, acquisition of funds, revision and supervision.
Funding
This work has been supported by FEDER/CM B2017/BMD-3688-Multitarget&View and EU H2020-INTERREG, 0551_PSL_6_E-NITROPROHEAR grants to I.V.-N; FEDER/MICIN, PID2020-115274RB-I00-THEARPY grant to I.V.-N. and S.M.C; and ACCI-CIBER-ISCIII ERP1PDI761 grant to S.M.C. S.M.C. holds a CIBER ISCIII researcher contract.
How to cite:
Cervantes, B., Bermúdez-Muñoz, J.M., Ruiz-García, C., Lassaletta, L., Contreras, J., Murillo-Cuesta, S., and Varela-Nieto, I. (2022).
Genetic, molecular and biochemical basis of auditory ageing: lessons from experimental models. Auditio, 6, e84.
https://doi.org/10.51445/sja.auditio.vol6.2022.0084
Correspondence
*Silvia Murillo-Cuesta
Instituto de Investigaciones Biomédicas
Alberto Sols CSIC-UAM
Arturo Duperier 4, 28029 Madrid, Spain
Email: smurillo@iib.uam.es
Editorial Office
Correction: Tomás Pérez Pazos.
Translation: Emma Goldsmith.
Production: Publicaciones Académicas