 1,2*, Alfredo Herrera1, Virginia Olivares1,2, Juan C. Maass3,4,5, Úrsula Zelada
1,2*, Alfredo Herrera1, Virginia Olivares1,2, Juan C. Maass3,4,5, Úrsula Zelada 6, Gloria Ribalta
6, Gloria Ribalta 7, Gabriela Vergara
7, Gabriela Vergara 1,8, Cristian Papuzinski
1,8, Cristian Papuzinski 9,10, Javiera Herrada
9,10, Javiera Herrada 4, Agustín D. Martínez11,12 and Helmut A. Sánchez11
4, Agustín D. Martínez11,12 and Helmut A. Sánchez11Research Articles
Sociodemographic and hearing profile of a population with sensorineural hearing loss in Chile
Elvira Cortese S. 1,2*, Alfredo Herrera1, Virginia Olivares1,2, Juan C. Maass3,4,5, Úrsula Zelada
1,2*, Alfredo Herrera1, Virginia Olivares1,2, Juan C. Maass3,4,5, Úrsula Zelada 6, Gloria Ribalta
6, Gloria Ribalta 7, Gabriela Vergara
7, Gabriela Vergara 1,8, Cristian Papuzinski
1,8, Cristian Papuzinski 9,10, Javiera Herrada
9,10, Javiera Herrada 4, Agustín D. Martínez11,12 and Helmut A. Sánchez11
4, Agustín D. Martínez11,12 and Helmut A. Sánchez11
1School of Speech-Language Pathology and Audiology, School of Medicine, Universidad de Valparaíso. Valparaíso, Chile / 2Speech-Language Pathology and Audiology Care Center, Universidad de Valparaíso. Valparaíso, Chile / 3Interdisciplinary Program of Physiology and Biophysics ICBM, Universidad de Chile. Santiago, Chile / 4Department of Otolaryngology, Hospital Clínico Universidad de Chile. Santiago, Chile / 5Department of Otolaryngology, School of Medicine, Clínica Alemana-Universidad del Desarrollo. Santiago, Chile / 6Department of Otolaryngology, Hospital Barros Luco Trudeau. Santiago, Chile / 7Department of Otolaryngology, Clínica Las Condes. Santiago, Chile / 8Hospital Dr. Gustavo Fricke. Viña del Mar, Chile / 9Department of Otolaryngology, Hospital Carlos van Buren. Valparaíso, Chile / 10Department of Medical Specialties, School of Medicine, Universidad de Valparaíso. Valparaíso, Chile / 11Interdisciplinary Center of Neuroscience of Valparaíso. Valparaíso, Chile / 12Neuroscience Institute, Faculty of Sciences, Universidad de Valparaíso. Valparaíso, Chile
 OPEN ACCESS
OPEN ACCESS
PEER REVIEWED
ORIGINAL RESEARCH
Abstract
Background. Evidence-based information on genetic sensorineural hearing loss (SNHL) in Latin America is limited, hindering the advancement of related clinical practice and the development of relevant healthcare policies in the field. This study describes the sociodemographic and clinical characteristics of a Chilean population with congenital, childhood, progressive or late-onset SNHL of unknown aetiology. Methods. This study had an observational, descriptive, cross-sectional design. Of 978 patients diagnosed with SNHL from seven health centres in Valparaíso and Santiago, 286 fulfilled the inclusion and exclusion criteria and 141 patients were contacted and agreed to participate. Results. Participants were aged 1-80 years; 50.7% of the sample was female. Most participants were in mainstream education and only 27% received special education. Most participants were part of a public health care insurance system (FONASA), while only 32% had a private health insurance system (ISAPRE). 1.5% of participants did not have any type of health insurance. 66% of the participants were registered in the National Disability Registry. The most frequent audiological profile was symmetrical, profound, bilateral SNHL with a sloping pattern. Median age at audiological diagnosis was 24.5 months (interquartile range (IQR),12-53) and age at first-time hearing-assistive-device (HAD) use was 30 months (IQR,13-69). 71% of HAD were funded by public resources. Conclusion. The age at audiological diagnosis of SNHL of suspected genetic causes is still far from international standards. These results are valuable for public health research and policy development, not only for the Chilean population, but also for other Hispanic communities and other upper-middle income countries.
Keywords
Hearing Loss; Sensorineural; Genetic Diseases; Inborn; Demography; Latin America
Clinical implications
The results of this study may help to better understand the sociodemographic and clinical variables of people with SNHL of unknown causes in Chile. A late diagnosis was found to be a major limitation on effective and timely access to hearing health services. The collection and management of clinical data must improve to increase the effectiveness and efficiency of current public healthcare policies. Further research is needed on the long-term impact of hearing health public policies in Chile.
Received: 04.03.2022 Revised: 09.05.2022 Accepted: 06.06.2022 Published: 01.09.2022
Edited by:
Raul Sanchez-Lopez,
University of Nottingham, United Kingdom
Reviewed by:
Sabina Storbjerg Houmøller,
University of Southern Denmark, Denmark.
Humberto Yévenes-Briones,
Autonomous University of Madrid, Spain.
Alberto Regordán,
Hospital de la Santa Creu i Sant Pau, Spain.
Introduction
Sensorineural hearing loss (SNHL) is one of the most common sensory disorders in humans, affecting 1 to 3 of every 1000 live births worldwide (Kenna et al., 2010). Little information is available about the prevalence and causes of this type of hearing loss, particularly in low-income countries (Haile et al., 2021). Indeed, in most cases of congenital, childhood, progressive or late-onset SNHL, the aetiology remains unknown (Berrettini et al., 1999; Billings & Kenna, 1999; Gürtler et al., 2017). Evidence shows that genetic causes account for about half of congenital SNHL cases and up to two-thirds of prelingual hearing loss cases (Haile et al., 2021; Kenna et al., 2010; Angeli et al., 2012). Early onset hearing loss has a profound negative impact on communication, affecting both educational and professional development. Hearing loss also has important consequences on social-emotional interactions, which can lead to isolation, depression and mental health deterioration (Asghari et al., 2017; Eskander et al., 2014; Instituto Nacional de Estadística de Chile, 2004). Timely diagnosis and early management of SNHL are crucial to reduce these negative effects (Joint Committee on Infant Hearing, 2019). Specifically, international guidelines recommend that a diagnosis should be made before the age of 3 months, with the goal of ensuring that intervention is initiated in all infants with hearing loss at no later than 3-6 months of age (Joint Committee on Infant Hearing, 2019). This goal can be achieved by designing specific public health policies (Brownson et al., 2009). However, in countries such as Chile where there are no public hearing-health policies for genetic screening and limited access to genetic testing, a timely and accurate audiological diagnosis is a challenge. The cause of SNHL remains unidentified in many cases, and a genetic origin can only be suspected (Arancibia et al., 2012). To improve the efficacy and efficiency of public health policies, it is important to have as much data available as possible for a given population (Brownson et al., 2009; Joint Committee on Infant Hearing, 2019). Sociodemographic data – age, sex, gender, socioeconomic level, educational level, occupation – are important, and more complex variables can also be described, such as standard of living, the effect of healthy living conditions on intellectual, social and global development, and factors associated with the state of health and disease, among others. Other important parameters relate to the efficiency of the health system and the long-term impact of existing public policies in a population (Brownson et al., 2009).
Clinical data also play a key role in providing a more complete overview of a given population. With respect to the SNHL population, access to specific, relevant clinical data (e.g., audiological diagnosis, age at diagnosis and SNHL aetiology), allows for a better understanding of this group’s health needs. Furthermore, having access to both sociodemographic and clinical data would help identify potential associations that could act as determinants of hearing health, thus facilitating the development of evidence-based preventive and treatment strategies. Consequently, public policies on hearing health must be based on specific and local data if they are to have a positive impact on SNHL prevention and early intervention (Brownson et al., 2009; World Health Organization, 2018). In Chile, we found few studies describing the clinical and sociodemographic characteristics of the population of patients with SNHL of unknown aetiology (Arancibia et al., 2012; Herrera, 2010). Therefore, the objective of this study was to describe the main sociodemographic and clinical characteristics of a group of patients with SNHL of probable genetic cause from seven health centres in urban areas of Santiago Metropolitan region and Valparaíso region.
Materials and Methods
This study had an observational, descriptive, cross-sectional design. Non-probabilistic sampling was used. The population consisted of 978 patients with congenital, childhood, progressive or late-onset SNHL, referred by otolaryngologists from seven participating centres. Of the total population, 286 patients fulfilled the inclusion criteria (i.e., patients aged 0-45 years with SNHL of unknown aetiology). To exclude environmental, non-genetic and syndromic causes of hearing loss and better target those cases of SNHL of unknown aetiology or probable genetic cause, the exclusion criteria included several conditions that might be associated with hearing loss, such as meningitis and prenatal infections (supplementary material, Table S1: inclusion and exclusion criteria). The sample selection stage was carried out by health professionals with expertise in clinical interviewing. The interview was conducted face-to-face in most cases or by telephone for participants living far away.
Of the 286 patients who met the inclusion criteria in the first stage of medical records reviews, 141 were contacted. All of them agreed to participate in the study. Three of these 141 participants were excluded during the clinical interview phase as they had one or more exclusion criteria that had not been documented in the medical record review. 145 patients could not be recruited into the study due to the COVID-19 pandemic. Finally, the sample consisted of 138 participants (Figure 1).
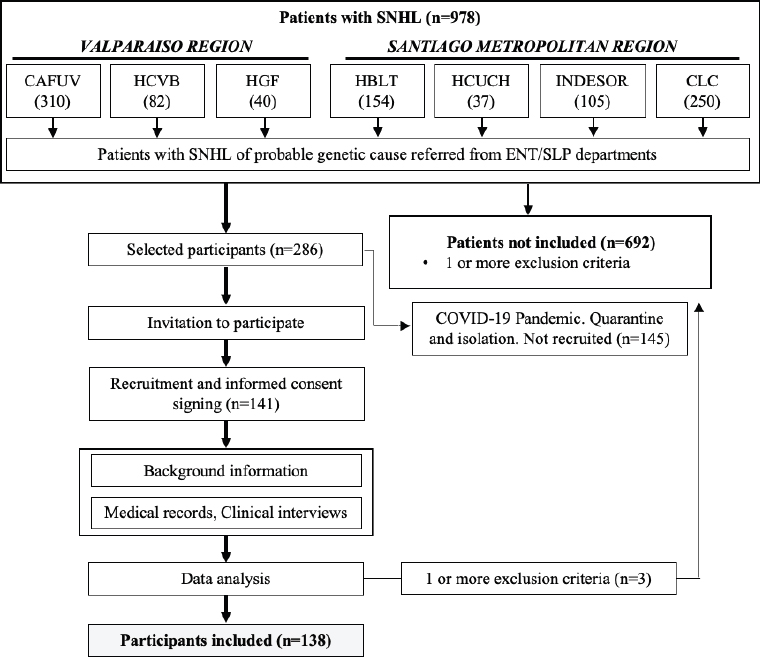
Figure 1. Flow chart for recruitment and selection of participants. SNHL: Sensorineural Hearing Loss. ENT: Otorhinolaryngologist. SLP: Speech-language pathologist. HCVB: Carlos Van Buren Hospital (Valparaíso); HCUCH: Universidad de Chile Hospital (Santiago); INDESOR: Jorge Otte School for the Deaf (Santiago); CLC: Las Condes Clinic (Santiago); HBLT: Barros Luco Trudeau Hospital (Santiago); HGF: Gustavo Fricke Hospital (Valparaíso); CAFUV: SLP Care Center - Universidad de Valparaíso (Valparaíso).
Participants were enrolled between September 2019 and March 2020. The otolaryngologists in charge of the respective units at the participating centres were contacted and invited to participate in the study. They were given information about the study to offer to potential participants. They provided a list of potential participants with SNHL of unknown cause. A group of healthcare professionals was in charge of sample selection, recruitment, and data collection by medical record review and structured clinical interview. We defined a set of sociodemographic variables, including sex, gestational age and birth weight, region of origin, monthly income, education and occupation. Clinical variables included comorbidities, family history of SNHL, audiological diagnosis by ear, age at audiological diagnosis, audiometric patterns of SNHL, SNHL symmetry and onset, age at first-time hearing-assistive-device (HAD) use, age at first cochlear implantation and HAD funding (supplementary material, Table S2: variables description). To reduce the possibility of memory bias, most of the data obtained from the interviews were cross-checked with the patients’ medical records. The interviews were administered mainly at the respective health centres or, in the case of participants living far away, by telephone. Participants signed a consent form before any study procedures were performed.
Data analysis was performed using Microsoft Excel (Microsoft, USA) and the free and open-source program JASP (University of Amsterdam, the Netherlands). We used skewness, Kurtosis and Shapiro-Wilk tests to check for normality. For data with n<=30, we applied non-parametric statistics. Non-parametric data results were reported as median (Mdn) and interquartile range (IQR) for continuous numerical variables and as frequencies and percentages for categorical variables.
Results
Sociodemographic variables
The age range of the participants was 1-80 years, 50.7% was female and 49.3% was male. Distribution by region showed that most participants came from the Santiago Metropolitan and Valparaíso regions, two of the most densely populated areas in Chile. We performed a data breakdown into smaller communes and conurbations for further analysis. Given the diversity of population sizes, the results were expressed as normalized values (number per 1000 inhabitants). The distribution by communes and association by conurbations showed a relative higher frequency of participants from the Quintero area in the Valparaíso region and from the Eastern area of Santiago in the Metropolitan region (Supplementary material. Figure S1). Communes without urban continuity were excluded from the conurbation analysis.
Table 1 summarizes the sociodemographic data. Most participants (76.3%) were students, most of whom were in mainstream education, with only 26.5% in special education systems. By occupation (participants aged 18 years or more), the majority were students, followed by professionals and individuals working in agricultural, fishery and handicraft activities. In the same adult group, 25% and 6% had completed undergraduate and graduate studies, respectively.
Table 1. Descriptive statistics of sociodemographic variables
Age |
Years |
Age. Median (IQR) |
14.5 (7.3 – 20.4) |
Age. Mean (Std. deviation) |
18.1 (15.4) |
Economic status |
Median in CLP2 pesos (IQR) |
Monthly income [n=128/138] |
222 500 (112 500 – 506 250) |
Sociodemographic variables |
Number of participants (%) |
Sex |
|
Female |
70 (50.7) |
Male |
68 (49.3) |
Region |
|
Valparaíso |
60 (43.5) |
O'Higgins |
8 (5.8) |
Araucanía |
7 (5.1) |
Santiago Metropolitan |
57 (41.3) |
Other |
6 (4.2) |
Current education level [n=106/138] |
Current [n=106] |
Adults without any formal educational qualifications |
2 (1.9) |
Unschooled under 4 years |
5 (4.7) |
Preschool |
20 (19) |
Elementary education (from first to eighth grade) |
48 (46) |
High school education / Technical |
17 (16) |
Unfinished High School |
1 (0.9) |
Undergraduate (University students) |
12 (11) |
Graduate (Master and PhD students) |
1 (0.9) |
Occupation, total |
[n=131/138] |
Students, total |
100 (76.3) |
– Mainstream school |
41/98 (41.8) |
– Mainstream school in Integration Programmes |
31/98 (31.6) |
– Special education (school for the deaf) |
26/98 (26.5) |
Professionals |
11 (8.3) |
Farmers, stockbreeders, fishermen |
3 (2.2) |
Retirees |
3 (2.2) |
Directors |
2 (1.5) |
Homemakers |
2 (1.5) |
Other |
10 (7.4) |
Health Insurance System (HIS) [n = 130/138] |
|
Public Health Insurance System (FONASA) |
86 (66) |
Private Health Insurance System (ISAPRE) |
42 (32) |
No Health Insurance System |
2 (1.5) |
Registered in national (Chilean) disability registry, n (%) [n=136/138] |
|
Yes |
90 (66.1) |
No |
46 (33.8) |
Hospital or participating centre, n (%) |
|
Barros Luco Trudeau Hospital (HBLT) |
25 (18.1) |
Universidad de Chile Hospital (HCUCH) |
3 (2.2) |
Jorge Otte School for the Deaf (INDESOR) |
11 (8.0) |
Las Condes Clinic (CLC) |
36 (26.1) |
Carlos Van Buren Hospital (HCVB)7 |
24 (17.4) |
Gustavo Fricke Hospital (HGF) |
7 (5.1) |
Speech-Language Pathology Care Center - Universidad de Valparaíso (CAFUV) |
32 (23.2) |
Monthly income expressed in Chilean pesos (CLP) showed a high dispersion, ranging from CLP 30 000 (approx. USD 40) to CLP 2.5 million (approx. USD 3 500). Participants from public centres had a median income of CLP 156 666 (IQR,100 000-300 250). The highest dispersion of values was observed in private centres. Regarding health insurance coverage, most participants were beneficiaries of the Public Health Insurance System (PHIS; FONASA) while only 32% were in the Private Health System (PHS; ISAPRE).
Audiological diagnosis and Hearing Assistive Devices (HAD)
Figure 2 shows age distribution at audiological diagnosis and the time interval from diagnosis to first-time HAD use in the sample. Audiological diagnosis was made in 76.3% of participants in the sample population before the age of 5 years. 69% were diagnosed before 3 years old and half the participants were diagnosed before the age of 24.5 months (IQR, 12-53.3). Notably, only 26.8% of the participants were diagnosed during the first year of life, of whom 56.8% were diagnosed before 6 months. Age at audiological diagnosis progressively decreased after Public Health Insurance Programme 1 (PHIP 1) was implemented in 2003 (Figure 2A).
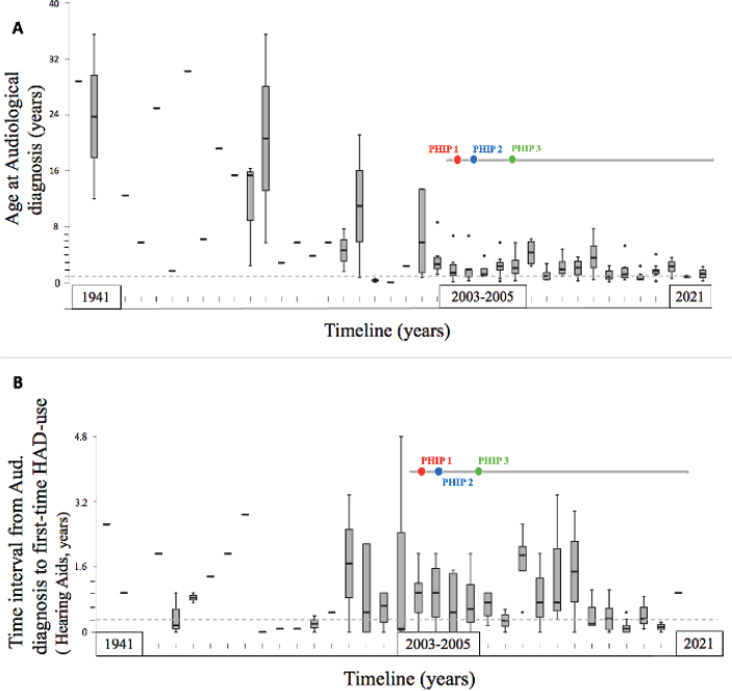
Figure 2. Age at audiological diagnosis and HAD use over time. A) Evolution of the age at audiological diagnosis over time. Box plots of median values, interquartile range, minimum and maximum values. n=138. Dotted reference line: 1 year. B) Time interval from the age at audiological diagnosis to age at first-time HAD use (Hearing Aid, HA) (n=116). Dotted reference line: 3 months. Red dot: Public Health Insurance Programme 1(PHIP 1): Cochlear Implant public programme of the Ministry of Health. FONASA MINSAL. Blue dot: Public Health Insurance Programme 2 (PHIP 2): SNHL Coverage for preterm infants (GES 59, MINSAL). Green dot: Public Health Insurance Programme 3 (PHIP 3): Coverage of SNHL in children under 2 years old (GES 77, MINSAL). Dotted lines represent an arbitrary value, close to what is suggested by competent international entities, for the variables described in A and B.
For the subgroup of participants diagnosed within the first year, the median time interval from audiological diagnosis to first-time HAD use was only 3 months (IQR, 0-8.0). A greater dispersion was observed for the time interval from first-time HAD use to first cochlear implantation, such that the median value was 30 months for the total sample (IQR, 10.0-87.0) and 20.5 months for the subgroup of participants diagnosed in the first year of life (IQR, 12.5-52.5). Also, shorter time intervals from the age at audiological diagnosis to first-time HAD use (Hearing aids, HA) were observed after Public Health Insurance Programme 1 (PHIP 1) was implemented in 2003 (Figure 2B).
The main clinical variables collected in this group of participants are summarized in Table 2. The most frequent audiological diagnosis was profound bilateral SNHL. The most frequent audiometric pattern was sloping, followed by residual, U-shaped and flat. Most participants had right-left hearing symmetry. In a few cases (13.8%), the onset of hearing loss followed a progressive course.
Table 2. Clinical audiological variables
Clinical Variable |
Frequency (%) |
|
|
|
|
Audiometric Pattern |
Right ear [n=100/138] |
Left ear [n=99/138] |
Sloping |
39 (39) |
41 (41.4) |
Residual |
22 (22) |
21 (21.2) |
U-shaped |
12 (12) |
14 (14.1) |
Flat |
12 (12) |
14 (14.1) |
Total deafness (anacusis) |
5 (5) |
2 (2.0) |
Rising |
2 (2) |
3 (3.0) |
Unclassifiable |
8 (8) |
4 (4.0) |
Total Hearing Assistive Devices in the sample, n (%) [n = 217] |
Right Ear |
Left Ear |
None |
25 (18.1) |
34 (24.6) |
Hearing aid |
63 (45.7) |
73 (52.9) |
Cochlear implant |
50 (36.2) |
31 (22.5) |
Right-Left Hearing Symmetry, n (%) [n=137/138] |
|
|
Symmetrical |
113 (82.5) |
|
Asymmetrical |
24 (17.5) |
|
Hearing loss onset, n (%) |
|
|
Not progressive |
119 (86.2) |
|
Progressive |
19 (13.8) |
|
HAD funding, n (%) [n = 209] |
|
|
Self-funded |
59 (28.2) |
|
PHIP GES 77: SNHL coverage in children under 4 years old |
57 (27.2) |
|
SPHIP: SNHL coverage in school-age children JUNAEB |
57 (27.2) |
|
SPHIP: FONASA MINSAL |
24 (11.4) |
|
SPHIP: Ricarte Soto |
5 (2.4) |
|
Other |
7 (3.5) |
|
In unilateral HAD users, cochlear implants (CI) were most frequent (19/27), with a predominance of right ear (RE). Unilateral hearing aid fitting was reported with lower frequency (8/27). In bilateral HAD users, bilateral hearing aid fitting was most frequent (45%), while bilateral CI were present only in 11% of the participants. The bimodal configuration (CI with hearing aid) was frequent (44%) with a predominance of CI in RE and hearing aid in LE.
Bilateral hearing aids (53%) were found mainly in public centres (66/95), followed by bimodal configuration (44%). Only two participants from public centres had bilateral CI, both funded by the PHIS. Moreover, in the private centres, the bimodal configuration was common (44%), followed by bilateral hearing aids (27.5%) and bilateral CI (27.5%). The majority of bilateral CI was found in private centres (80%), 75% of which were funded by the PHIS, with the remaining 25% were funded by private schemes or self-funding.
Students were the most frequent users of bilateral HAD (83%) and almost all these users were in preschool, elementary or secondary education (82%). In the group of students with higher and graduate education, bilateral HAD use predominated (69%). Bimodal configuration and bilateral hearing aids were used most frequently among participants in mainstream education (48% and 41%, respectively) and in mainstream schools with integration programmes (41% and 51%, respectively). The frequency of bilateral HAD users was lower in special education than in mainstream systems (with or without integration programmes), with eight participants having bilateral hearing aids, six with bimodal configuration and two with bilateral CI. Of a total of 217 HADs in the sample, the majority were subsidized by PHIPs.
Audiological tests
Of the 166 audiological tests collected in the sample, 65.7% were pure-tone audiometry and 34.3% were objective electrophysiological tests, such as auditory brainstem responses (ABRs) with click-type stimuli (23.5%), tone-burst stimuli (6%), and auditory steady-state responses (ASSR) (4.8%). Of note, 50% of the participants diagnosed before 3 years old only underwent pure-tone audiometry and 44% had at least one objective electrophysiological test in their medical records. The summary of the results is shown in Table 3.
Table 3: Summary of the audiometric results
Audiometric thresholds |
Number of observations |
Right ear Median (IQR) |
Left ear Median (IQR) |
Pure-tone audiometry [dB HL] |
|||
Pure-tone average (0.5, 1, 2, 4 kHz) |
110 |
98.0 (69.5-110.8) |
95.0 (68.0-110.0) |
Auditory brainstem responses (ABR) [dB nHL] |
|||
Click |
39 |
110.0 (100.0-110.0) |
110.0 (100.0-110.0) |
Tone burst 0.5 kHz |
9 |
110.0 (100.0-110.0) |
110.0 (80.0-110.0) |
Tone burst 1 kHz |
4 |
110.0 (90.0-110.0) |
110.0 (90.0-110.0) |
Tone burst 2 kHz |
4 |
110.0 (100.0-110.0) |
110.0 (107.3-110.0) |
Tone burst 4 kHz |
4 |
110.0 (105.0-110.0) |
110.0 (100.0-110.0) |
Auditory Steady-State Responses (ASSR) [dB eHL] |
|||
Carrier tone 0.5 kHz |
10 |
72.5 (65.0-96.3) |
77.5 (67.5-90.0) |
Carrier tone 1 kHz |
10 |
80.0 (70.0-90.0) |
80.0 (75.0-95.0) |
Carrier tone 2 kHz |
10 |
87.5 (75.0-98.8) |
87.5 (80.0-122.5) |
Carrier tone 4 kHz |
10 |
82.5 (66.3-100.0) |
82.5 (75.0-98.8) |
Discussion
Principal findings
The results of this study provide a general overview of the sociodemographic and hearing profile of people with SNHL of probable genetic cause from two of the most densely populated areas in Chile (López V. et al., 2012, Rodríguez et al., 2003).
The most frequent audiological profile in the sample was symmetrical, profound, bilateral SNHL with a sloping pattern (Table 1 and supplementary table S2). However, three of the seven participating centres were cochlear implantation facilities, which might have influenced the observed hearing loss severity and/or audiometric pattern. Despite this, the four non-implantation centres together accounted for over a third of participants (39%), and the same audiological diagnosis distribution was observed at these centres. Although the high variability of genetic alterations related to hearing loss makes it difficult to correlate a specific phenotype with a particular mutation (Cabanillas Farpón & Cadiñanos Bañales, 2012, Sloan-Heggen et al., 2016), severe to profound SNHL and bilateral symmetrical presentation appear to be the most dominant clinical features related to genetic deafness (Billings & Kenna, 1999; Cabanillas Farpón & Cadiñanos Bañales, 2012). Additionally, it is known that most genetic mutations related with SNHL (GJB2, SLC26A4, CDH23, MY015A, OTOF, TMC1 and others) show an autosomal recessive inheritance pattern (Mittal et al., 2018). Moreover, the presence of X-linked mutations (e.g., mutations in the POU3F4 gene and mutations associated with Alport syndrome) and those affecting mitochondrial genes (e.g., mutations in the MT-RNR1 gene) may also explain the presence of hearing loss. Since we did not recruit patients with syndromic phenotypes or patients with a history of antibiotic exposure, the patients in our study are unlikely to carry these inheritance patterns. However, Martini et al. (1997) reported that recessive forms of non-syndromic genetic hearing loss usually have a profound, symmetrical and non-progressive pattern, while dominant forms tend to be less severe, with a postnatal or progressive onset. Also, it has been reported that symmetry may be a key pattern in hereditary SNHL. Several authors have acknowledged the difficulty in subclassifying recessive forms of non-syndromic hearing loss using an audiometric criterion, while others have suggested that in both congenital and rapid prelingual onset hereditary hearing loss, the most common phenotypic presentation is profound SNHL with a sloping pattern (Liu & Xu, 1994; Paparella et al., 1975).
Median age at audiological diagnosis in our study was 24.5 months (IQR, 12-53 months), which is late in comparison with international guidelines that recommend that identification of hearing loss and audiological intervention should not exceed 3 to 6 months of age. Also, the suggested age for cochlear implantation is around 12 months in infants with severe to profound hearing loss, including those with auditory neuropathy who progress poorly with hearing aids (Joint Committee on Infant Hearing, 2019). Our results could be partially explained by the type of hearing loss onset, as almost 14% of participants had a history of progressive SNHL. Retamal-Walter et al. (2020) suggested that a delay in diagnostic confirmation could also be explained by limited knowledge about early auditory diagnosis and intervention among neonatal and paediatric health care teams. Harrison et al. (2003) found that the median age at audiological diagnosis was later in children with SNHL with unidentified causes than in those with SNHL with identified causes, with a median of 25.0 months, as corroborated in this study. The later age at diagnosis in Chile compared with recommended standards may be due to the lack of a universal screening programme and/or the scarce availability of otolaryngologists, making it hard to reach a diagnosis during an infant’s first months of life, which in turn leads to delayed access to treatment and to assistive devices and aural rehabilitation. All these delays have a negative impact on children's oral language acquisition and their global development (Asghari et al., 2017; Eskander et al., 2014; Instituto Nacional de Estadística de Chile, 2004). We observed that the median age at audiological diagnosis dropped to around 12 months at two participating centres, following two decades of implementing recommended standards, being pioneers in cochlear implantation and with universal neonatal hearing screening in Chile (Albertz et al., 2013). This highlights the need to follow international standards recommended by leading organizations in this field, to reduce the gap in obtaining access to hearing health care.
Finally, we observed that only 44% of participants diagnosed before 3 years of age had objective audiological tests available in their medical records. This finding shows non-fulfilment of requirements for paediatric audiological diagnosis, which include at least one objective test with frequency specific measurements, such as tone-burst BERA or ASSR (Gobierno de Chile - Ministerio de Salud, 2013; Joint Committee on Infant Hearing, 2019). This situation may be partially explained by several factors. First, objective tests such as BERA and ASSR are expensive compared to pure-tone audiometry; therefore, access to these procedures is limited at public health care centres. Furthermore, 25% of the above group was diagnosed before the year 2000, when public policies on hearing health had not been implemented in Chile and paediatric hearing assessment was mainly performed through subjective tests. Also, in this study a small proportion of participants had a history of progressive hearing loss. In some cases, SNHL assessment was not only performed at the main treatment centre, but also at centres outside the public system; therefore, some patient data may not have been incorporated into a single medical record. Also, most public health centres rely on paper-based medical records, hindering data exchange among centres and sometimes leading to loss of patient medical information. Consequently, there is a clear need to implement an integrated information system that allows people with SNHL to access a better hearing health system.
Future perspectives
The advantages of improving the aetiological diagnosis of hearing loss are well known (Alford et al., 2014; Gürtler et al., 2017; Wiley et al., 2011, Urbančič et al., 2020). For example, patients are informed of the prognosis and evolution of their hearing loss, receive guidance regarding comorbidities that may require referral for specific management, and in general they can plan future health and educational needs (Alford et al., 2014). There is now a body of evidence that informs the genetic testing strategies to achieve this goal, through aetiological diagnosis (Urbančič et al., 2020). Targeted genetic sequencing and deafness-associated gene panels have been widely used for this purpose (Tayoun et al., 2016). However, to date, the NCBI Gene database contains a registry of more than 200 genes, some 1300 variants, associated with SNHL and more than 100 genes related to non-syndromic deafness (https://www.ncbi.nlm.nih.gov/gene), and therefore the success of these techniques to identify the cause of deafness is limited. Despite this, exome and whole genome sequencing techniques can now analyse all genes and identify any pathogenic variant associated with hearing loss (Retterer et al., 2016), thus deciphering the genetic heterogeneity of deafness. The main drawback of these new-generation methods lies in their high cost.
In Chile, the lack of universal genetic screening has led to many cases of hearing loss being left over time without an aetiological diagnosis, as in the case of all the participants in this study for whom a genetic origin of their hearing loss can only be suspected. Including rapid genetic testing as public policy for universal screening would improve this scenario. Experts in this field (Brownson et al., 2009) believe that new health policies can be built only with an in-depth understanding of the target population. Therefore, the characterization of a population with suspected genetic hearing loss in Chile would allow a more efficient targeting of health care resources, definition of potential risk factors and identification of possible correlations between variables. This, together with the development of molecular diagnostic tools that complement and expand the coverage of hearing screening programmes, would help develop improved strategies for diagnosis, prevention, and treatment, progressively reducing the impact of hearing loss in Chile. Finally, regardless of the method used to improve screening and diagnostics, genetic counselling strategies must be provided by trained health professionals. (Alford et al., 2014; Cooke-Hubley & Maddalena, 2011).
Limitations
Although all patients with a clinical history suggesting any environmental, non-genetic or syndromic causes were excluded from the sample, it is possible that some participants in this study had SNHL of a non-genetic origin.
The sample size of this investigation was reduced by the impact of COVID-19 on study conduct, such that it limited the recruitment of new potential participants and collection of their medical information.
The design of this study was purely descriptive. Most participants came from the Valparaíso and Santiago metropolitan regions. Future studies are needed in this field to cover a larger population of people with congenital, childhood, progressive or late-onset SNHL, using more complex methodologies (e.g., collecting biological samples), which may provide answers to new questions.
Conclusions
The information collected in this study represents the first report of the sociodemographic and clinical characteristics of a Chilean population with SNHL of probable genetic cause. The research process showed how public hearing health policies have influenced hearing health in Chile in the last decades and the data collected revealed some limitations to effective and timely access to hearing health services in Chile. Specifically, the age at audiological diagnosis of SNHL of suspected genetic causes is still far from international standards. This highlights the need for further research on the long-term impact of public policies in Chile. The sociodemographic and clinical data presented in this study will be a useful tool for public health research and policy development, not only for the Chilean population, but also for other Hispanic communities and upper-middle income countries.
References
Albertz, N., Cardemil, F., Rahal, M., Mansilla, F., Cárdenas, R., & Zitko, P. (2013). Programa de tamizaje universal e intervención precoz (PTUIP) en hipoacusia sensorioneural bilateral congénita. Tarea pendiente desde la perspectiva de políticas públicas de salud en Chile. Revista Médica de Chile, 141(8), 1057–1063. https://doi.org/10.4067/S0034-98872013000800013
Alford, R. L., Arnos, K. S., Fox, M., Lin, J. W., Palmer, C. G., Pandya, A., Rehm, H. L., Robin, N. H., Scott, D. A., & Yoshinaga-Itano, C. (2014). American college of medical genetics and genomics guideline for the clinical evaluation and etiologic diagnosis of hearing loss. Genetics in Medicine, 16(4), 347–355. https://doi.org/10.1038/gim.2014.2
Angeli, S., Lin, X., & Liu, X. Z. (2012). Genetics of Hearing and Deafness. Anatomical Record, 295(11), 1812–1829. https://doi.org/10.1002/ar.22579
Arancibia, S., Margarita, R., N, R., Farfán, R., Corina, A., P, M., Cifuentes, O., & Lucía. (2012). Frequency of the 35delG mutation of GJB2 (connexin 26) in a sample of deaf school children in Santiago. Revista de Otorrinolaringología y Cirugía de Cabeza y Cuello, 72(1), 7–14. https://doi.org/10.4067/S0718-48162012000100002
Asghari, A., Farhadi, M., Daneshi, A., Khabazkhoob, M., Mohazzab-Torabi, S., Jalessi, M., & Emamjomeh, H. (2017). The Prevalence of Hearing Impairment by Age and Gender in a Population-based Study. Iran J Public Health, 46(9), 1237–1246. https://pubmed.ncbi.nlm.nih.gov/29026790/
Berrettini, S., Ravecca, F., Sellari-Franceschini, S., Matteucci, F., Siciliano, G., & Ursino, F. (1999). Progressive sensorineural hearing loss in childhood. Pediatric neurology, 20(2), 130–136. https://doi.org/10.1016/s0887-8994(98)00123-4
Billings, K. R., & Kenna, M. A. (1999). Causes of Pediatric Sensorineural Hearing Loss Yesterday and Today. Arch Otolaryngol Head Neck Surg, 125, 517–521. https://doi.org/10.1001/archotol.125.5.517
Brownson, R. C., Chriqui, J. F., & Stamatakis, K. A. (2009). Policy, Politics, and Collective Action; Understanding Evidence-Based Public Health Policy. American Journal of Public Health, 99(9), 1576–1583. https://doi.org/10.2105/AJPH.2008.156224
Cabanillas Farpón, R., & Cadiñanos Bañales, J. (2012). Hipoacusias hereditarias: Asesoramiento genético. Acta Otorrinolaringologica Espanola, 63(3), 218–229. https://doi.org/10.1016/j.otorri.2011.02.006
Chile, I. N. (2004). Primer Estudio Nacional de la Discapacidad—Deficiencias Auditivas y Trastornos Severos de la Comunicación. https://n9.cl/pnohm
Cooke-Hubley, S., & Maddalena, V. (2011). Access to genetic testing and genetic counseling in vulnerable populations: The d/Deaf and hard of hearing population. J. Community Genet, 2, 117–125. https://doi.org/10.1007/s12687-011-0047-z
de Chile - Ministerio de Salud., G. (2013). Guía clínica AUGE, tratamiento de la Hipoacusia moderada en menores de 2 años. https://web.archive.org/web/20220423012748/https://www.minsal.cl/portal/url/item/de429df07a91ca3ce040010165017ea0.pdf.
Eskander, A., & Papsin, B. C. (2014). Screening infants for hearing impairment in Canada. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne, 186(14), 1048–1049. https://doi.org/10.1503/cmaj.131685
Gürtler, N., Gysin, C., Schmid, N., Pieren, C., Vischer, M., Schumacher, S., Oppermann, P., Leuba, D., & Veraguth, D. (2017). Bilateral congenital deafness: What investigations should be performed? A qualitative descriptive review. Swiss Med Wkly, 147, 14416. https://doi.org/10.4414/smw.2017.14416
Haile, L. M., Kamenov, K., Svitil Briant, P., Orji, A. U., Steinmetz, J. D., Abdoli, A., Abdollahi, M., Abu-Gharbieh, E., Afshin, A., Ahmed, H., Ahmed Rashid, T., Akalu, Y., Alahdab, F., Mashhour Alanezi, F., Alanzi, T. M., Hamad, H., Ali, L., Alipour, V., & Al-Raddadi, R. M. (2021). Hearing loss prevalence and years lived with disability, 1990–2019: Findings from the Global Burden of Disease Study 2019. The Lancet, 397(10278), 996–1009,. https://doi.org/10.1016/S0140-6736(21)00516-X.
Harrison, M., Roush, J., & Wallace, J. (2003). Trends in Age of Identification and Intervention in Infants with Hearing Loss. Ear and Hearing, 24(1), 89–95. https://doi.org/10.1097/01.AUD.0000051749.40991.1F
Herrera, V. (2010). Estudio de la población Sorda en Chile: Evolución histórica y perspectivas lingüísticas, educativas y sociales. Revista Latinoamericana de Educación Inclusiva, 4(1), 211–226. http://repositoriocdpd.net:8080/handle/123456789/1916
Infant Hearing, T. J. C. (2019). Year 2019 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. Journal of Early Hearing Detection and Intervention, 4(2), 1–44. https://doi.org/10.15142/fptk-b748
Kenna, M. A., Feldman, H. A., Neault, M. W., Frangulov, A., Wu, B. L., Fligor, B., & Rehm, H. L. (2010). Audiologic phenotype and progression in GJB2 (connexin 26) hearing loss. Archives of Otolaryngology - Head and Neck Surgery, 136(1), 81–87. https://doi.org/10.1001/archoto.2009.202
Liu, X., & Xu, L. (1994). Nonsyndromic hearing loss: An analysis of audiograms. Annals of Otology, Rhinology & Laryngology, 103(6), 428–433. https://doi.org/10.1177/000348949410300602
López, V., G., P., Y, A., Pittaluga, P., E., B., M, L., Godoy, B., C., Repetto, L., & G, M. (2012). Evaluación de mutaciones en los genes GJB2 y GJB6 en pacientes con sordera congénita identificados mediante screening neonatal. Revista Chilena de Pediatría, 83(2), 154–160. https://doi.org/10.4067/S0370-41062012000200006
Martini, M., Milani, M., Rosignoli, M., Mazzoli, M., & Prosser, S. (1997). Audiometric Patterns of Genetic Non-syndromal Sensorioneural Hearing Loss. Audiology, 36, 228–236. https://doi.org/10.3109/00206099709071975
Mittal, R., Patel, A. P., Nguyen, D., Pan, D. R., Jhaveri, V. M., Rudman, J. R., Dharmaraja, A., Yan, D., Feng, Y., Chapagain, P., Lee, D. J., Blanton, S. H., & Liu, X. Z. (2018). Genetic basis of hearing loss in Spanish, Hispanic and Latino populations. Gene, 647, 297–305. https://doi.org/10.1016/j.gene.2018.01.027
Organization, W. H. (2018). Hearing loss is on the rise! https://cdn.who.int/media/docs/default-source/documents/world-hearing-day-2018-infographic.pdf?sfvrsn=54ccef8d_12
Paparella, M., Hanson, D., Rao, K., & Ulvestad, R. (1975). Genetic sensorineural deafness in adults. Trans.Amer.Otol.Soc, 63, 116–129. https://doi.org/10.1177/000348947508400404
Presidencia, M. S. G. (1999). Sobre protección de la vida privada. Biblioteca del Congreso Nacional de Chile. Ley de Chile número 19.628. http://bcn.cl/2l3au
Retamal-Walter, F., Gómez-Lombardi, A., & Martínez-Zelaya, G. (2020). Conocimientos, conductas y toma de decisiones de médicos pediatras sobre hipoacusia infantil en tres hospitales del Servicio de Salud Viña del Mar-Quillota. Rev. Otorrinolaringol. Cir. Cabeza Cuello, 80(4), 477–487. https://doi.org/10.4067/S0718-48162020000400477
Retterer, K., Juusola, J., Cho, M. T., Vitazka, P., Millan, F., Gibellini, F., Vertino-Bell, A., Smaoui, N., Neidich, J., Monaghan, K. G., McKnight, D., Bai, R., Suchy, S., Friedman, B., Tahiliani, J., Pineda-Alvarez, D., Richard, G., Brandt, T., Haverfield, E., & Bale, S. (2016). Clinical application of whole-exome sequencing across clinical indications. Genetics in Medicine, 18(7), 696–704. https://doi.org/10.1038/gim.2015.148
Rodríguez, J., Varela, T., Claps, D., Mires, L., Rodriguez, M., Tacla, O., Villalón, G., & Villalón, J. (2003). Comisión Nacional del XVII Censo de población y vivienda. https://www.ine.cl/docs/default-source/censo-de-poblacion-y-vivienda/publicaciones-y-anuarios/2002/sintesiscensal-2002.pdf
Sloan-Heggen, C. M., Bierer, A. O., Shearer, A. E., Kolbe, D. L., Nishimura, C. J., Frees, K. L., Ephraim, S. S., Shibata, S. B., Booth, K. T., Campbell, C. A., Ranum, P. T., Weaver, A. E., Black-Ziegelbein, E. A., Wang, D., Azaiez, H., & Smith, R. J. H. (2016). Comprehensive genetic testing in the clinical evaluation of 1119 patients with hearing loss. Human Genetics, 135(4), 441–450. https://doi.org/10.1007/s00439-016-1648-8
Tayoun, A. N. A., Turki, S. H., Oza, A. M., Bowser, M. J., Hernandez, A. L., Funke, B. H., Rehm, H. L., & Amr, S. S. (2016). Improving hearing loss gene testing: A systematic review of gene evidence toward more efficient next-generation sequencing-based diagnostic testing and interpretation. Genetics in Medicine, 18(6), 545–553. https://doi.org/10.1038/gim.2015.141
Urbančič, N. B., Battelino, S., Tesovnik, T., & Podkrajšek, K. T. (2020). The importance of early genetic diagnostics of hearing loss in children. Medicina, 56(9), 471. https://doi.org/10.3390/medicina56090471
Wiley, S., Arjmand, E., JareenMeinzen-Derr, & Dixon, M. (2011). Findings from multidisciplinary evaluation of children with permanent hearing loss. International Journal of Pediatric Otorhinolaryngology, 75(8), 1040–1044. https://doi.org/10.1016/j.ijporl.2011.05.019
Author contributions
EC, JCM, HS and AM study conception, funding management. EC, leading data analysis, manuscript writing and English adaptation before submission. VO, AH and HS, research data acquisition when acquisition required significant judgment or intellectual input, writing, editing and English adaptation before submission. JCM, statistical advice, analysis and interpretation of research data. JH, assistance in study and data collection. UZ, GR, GV and CP, providing access to the patients and medical records, critical review of the manuscript.
Conflict of interest
The authors declare that they have no potential or existing conflicts of interest, as follows: no associations with commercial entities that provided support or could have an interest in the work reported in the submitted manuscript; no financial associations involving family; and no other relevant non-financial associations.
Funding
This work was supported by a National Fund for Health Research - ANID Government of Chile (FONIS/FONDEF SA18I0194) awarded to E. Cortese, J.C. Maass, H.A. Sánchez and A. Martínez; and by the Chilean Millennium, Interdisciplinary Center of Neuroscience of Valparaíso (Grant P09-022F) to A.D. Martínez and H. Sánchez.
Ethics approval and consent to participate
This study was approved by CEC-UV (Protocol#CEC184-18), CEC-UChile (Protocol#171-2018, Act #202) and by the Institutional Review Boards (IRBs) from the respective participating centres. All participants signed a written informed consent. All the collected information was stored in compliance with personal data protection regulations and with Chilean laws in force, http://bcn.cl/2l3au (Ministerio Secretaría General de la Presidencia, 1999), and with the principles expressed in the Declaration of Helsinki.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, E.C. The data are not publicly available due to ethical restrictions as they contain information that could compromise the privacy of research participants.
Acknowledgements
We thank Lorena Cabezas (SLP) (Universidad de Valparaíso and Van Buren Hospital. Valparaíso, Chile) for her valuable collaboration with the logistics in the first stage of the study. We also thank the "Corporación Hipoacusia de Chile" for their contribution in the approach to the population of people with Hearing Loss. We are also grateful to Dr. Chiayu Q. Chiu (Universidad de Valparaíso) for her assistance in the final editing and English language support before submission.
Complementary material
The complementary material is available at: https://journal.auditio.com/auditio/e82/suppl
How to cite:
Cortese, E., Herrera, A., Olivares, V., Maass, J.C., Zelada, U., Ribalta, G., Vergara, G Cristian Papuzinski, C., Herrada, J., Martínez, A.D., & Sánchez, H.A. (2022).
Sociodemographic and hearing profile of a population with sensorineural hearing loss in Chile. Auditio, 6, e82.
https://doi.org/10.51445/sja.auditio.vol6.2022.0082
Correspondence
*Elvira Cortese S.
Angamos 655, Reñaca, Viña del Mar. School of Speech-Language Pathology and Audiology, School of Medicine, Universidad de Valparaíso. Valparaíso, Chile. Angamos 680, Reñaca, Viña del Mar. Speech-Language Pathology and Audiology Care Center, Universidad de Valparaíso. Valparaíso, Chile.
email: elvira.cortese@uv.cl
Oficina Editorial
Correction: Emma Goldsmith.
Translation: Tomás Pérez Pazos.
Producción: Publicaciones Académicas.