 1,*
1,*Research Articles
Preliminary results of the enriched acoustic environment as personalized sound-therapy for tinnitus
EAE as tinnitus sound therapy
1Instituto de Tecnologías Físicas y de la Información (ITEFI), Consejo Superior de Investigaciones Científicas (CSIC), Serrano 144, 28006 Madrid, Spain
 OPEN ACCESS
OPEN ACCESS
PEER REVIEWED
RESEARCH REPORT
Summary
The objective of this article is to describe the basics of a sound therapy, the Enriched Acoustic Environment (EAE), for selective stimulation of tinnitus patients. The sound stimulus consists of a sequence of random frequency tones, which can be of three types (pip, burst or gamma) within the audible frequency band, each with an amplitude directly proportional to the subject's hearing loss at that frequency. Pip tone and burst tone sequences have already demonstrated their effectiveness in restoring the tonotopic map of animals previously exposed to traumatizing noise and in restoring hypersensitivity to a group of patients with hyperacusis, respectively. The gammatone sequences, the fundamentals of which are described in this article, are an original proposition. The resulting tone series sequentially stimulates the subject's hearing system by compensating for hearing loss, which is one of the causes that trigger tinnitus.
Keywords
Tinnitus, sound therapy, enriched acoustic environment
Clinical implications
The article describes a personalized sound therapy for tinnitus that can be applied in the specialist's own clinic. To do this, the patient is received in the clinic, the audiometry of both ears is measured, and the stereo EAE stimulus is designed, which is delivered to the patient in mp3 format to be heard one hour a day for four months. For follow-up, the patient is required a monthly THI that can be filled in at home and sent by email.
Received: 15.07.2021 Revised: 18.08.2021 Accepted: 24.08.2021 Published: 13.10.2021
Edited by:
Helia Relaño-Iborra
Technical University of Denmark
Revised by:
Patricia Vázquez-González
Fundación Vinjoy, Spain
Juan Manuel Espinosa-Sanchez
Universidad de Granada, Spain
Maria Teresa Heitzmann Hernández
Clínica Universitaria de Navarra, Spain
1. Introduction
Some people have occasionally noticed buzzing in the ears that does not come from outside the hearing system and may be associated with some reversible cause, such as exposure to very high noise (music concert, fireworks) or a transitory disturbance of the ear (Eggermont, 2012). However, in a percentage of the population, this inner noise, once it has originated, does not cease. The perception of sound in the absence of an external acoustic source is called tinnitus and is more prevalent in the older population (12% in those over 60 years) than in the younger population (5% between 20 and 30 years; Baguley et al., 2013). In 1-2% of the population, tinnitus severity is such that it affects quality of life, causing discomfort, sleeping problems, stress, anxiety, or depression. In the U.S. between 25 and 52 million people suffer from tinnitus (Jastreboff and Jastreboff, 2000), while in Europe, an estimated 5 million people experience moderate or severe buzz (Vio and Holmes, 2015). In Spain, 17% of patients in an ENT clinic reported this type of noise (Herráiz and Hernández Calvín, 2002), while in 1% of patients, tinnitus is highly invalidating (López González, 2010).
Thus, chronic tinnitus (long or continuous perceived for more than three months) can lead to a significant reduction in the quality of life of the sufferer. Damage to the peripheral hearing system of a group of laboratory animals from exposure to traumatizing noise has been shown to lead to functional changes in their cortical tonotopic map (Noreña and Eggermont, 2005). The ability to change the cortical and subcortical hearing system is called auditory plasticity. Overexposure to noise, aging, ear infection, ototoxicity, and Ménière's disease, among others, result in peripheral hearing loss (HL), leading to defferentation of the central auditory system, which may be a possible trigger for tinnitus.
HL is recognized as a risk factor for tinnitus (Tan et al., 2013). However, there are many individuals with HL who do not suffer from tinnitus. In patients with sensorineural HL, the prevalence of tinnitus is higher, and its pitch is higher (>3 kHz) than in those with conductive HL, in which the pitch is lower (< 1 kHz; Cuesta and Cobo, 2018). It then appears that the relationship of tinnitus with HL is more complex and that other factors, such as alterations in the synapses between hair cells and the fibres of the auditory nerve, may contribute to the appearance of tinnitus (Paul et al., 2017).
The neural substrates of tinnitus suggest several approaches to modify neural processing and change its properties, thus obtaining some relief. Although there is currently no specific drug to cure tinnitus, neurophysiological or psychological approximations may be used to reduce its effects. Neurophysiological-based treatments include substitution methods to compensate for lost activity at the cochlea output, via specially designed acoustic environments, or amplifying the sound environment in the lost frequency ranges by hearing aids or some other sound-generating device. Psychological approaches are based on neurophysiological models of tinnitus or derived from paradigms of treatment in people with depression. It is usually applied in conjunction with some sound therapy (Jastreff, 2015).
Plasticity can be used to reduce tinnitus perception by appropriately stimulating the auditory pathway (Schaette & Kempter, 2006). This has led to different sound therapies, the main objective of which is to produce habituation (Jastreff, 1990) or residual inhibition (Henry, 2016). Habituation occurs by eliminating connotations that negatively affect quality of life, acting more on limbic and autonomous systems, combining therapeutic counseling (habituation to reaction) and the use of sound therapy (habituation to perception). The subject must go through these two stages to get to ignore tinnitus (habituation). Masking tinnitus can make its perception disappear. Once the stimulus stops, it takes some time to experience it again, known as residual inhibition, which can last a variable time (from a few seconds to hours). The relationship between the parameters of sound stimulation, such as spectrum, intensity and duration and their effect on tinnitus, is not well known (Henry, 2016), which has led to the proposal of a large number of sound therapies (Pienkowski, 2019).
The main objective of this paper is to lay out the basis for sound therapy based on the patient's HL curves, especially suitable for tinnitus. This sound therapy, called the Enhanced Acoustic Environment (EAE), consists of a sequence of random frequency tones within the auditory band (the frequency band in which HL is measured) and an amplitude directly proportional to the hearing loss value at that frequency.
2. Materials and methods
Noreña and Eggermont (2005) have already demonstrated that a group of animals previously exposed to traumatizing noise that altered their cortical tonotopic map, when they were presented with a rich acoustic environment consisting of a random sequence of pip tones, recovered their normal tonotopic map. Noreña and Chery-Croze (2007), on the other hand, used similar sound therapy to reverse the hypersensitivity suffered by a group of subjects with hyperacusis. In this case, the stimulus consisted of a sequence of burst tones of random frequency and amplitude proportional to the hearing loss. In fact, this burst tone sequence was a variant of the pip tone sequence used by Noreña and Eggermont in their animal model. The properties of pip and burst tones are discussed in more detail below, and the introduction of a new sequence, gammatones, is motivated.
2.1. Pip tone sequences
Pip tones are shaped (Noreña and Eggermont, 2005)
where α and γ are two constants that determine the tone waveform, and therefore its spectrum. Therefore, a pip (STP) tone sequence has the form
Where fm is the frequency of the randomly chosen pip tones within the band of interest, τm is the delay of each, or interlatency between the tones, and Am is the amplitude of each tone, given by
HL(fm) is the value of the hearing loss at the frequency fm. Thus, each of the pip tones is determined by the parameters (α,γ) and by the shooting rate (τ, number of tones per second). Most published works use γ= 3. For pulse rate, a fast one (20 per second) and a slow one (1 per second) are usually used.
2.2. Burst tone sequences
Burst tones are defined by the equation:
Where w(t) is a temporary window (rectangular, Hanning, Hamming, etc.). Therefore, a burst tone sequence would be
Where fm, τm and Am have the same meaning as in the Eqs. (1-3). Noreña and Chery-Croze (2007) used a rectangular window, w(t), with linear ramps of 5 ms up and down.
2.3. Gammatone sequences
Both pip and burst tones selectively stimulate the auditory pathway. However, the pip tone spectrum, determined by the parameter pair (α, γ), is more similar to the response curve at frequencies in different parts of the auditory pathway (the basilar membrane, for example). On the other hand, this frequency response curve depends on the species. Therefore, optimal EAE therapy for tinnitus would be one based on pip tone sequences with a pair of parameters (α, γ) appropiately defined for the human hearing system. Gamma filters are precisely those pip tones, with variables (α, γ) appropriate for the human hearing system. Specifically, the equation for a gammatone is
When the gammatone is transformed to the frequency domain, a gamma filter of order n, bandwidth b, central frequency f0, and phase ϕ is obtained. Patterson (1994) demonstrated that a human hearing filter is obtained with a gamma filter of order n= 4 and equivalent rectangular bandwidth (ERB), also called the "critical band", given by
A bank of gamma filters distributed along the frequency axis is often used to simulate the movement of the basilar membrane. Finally, the following equation is obtained for the gammatone sequence
Where fm, τm and Am have already been defined in section 2.1, bm is the bandwidth equivalent to the frequency fm obtained using Eq. (7), and is a normalization factor.
2.4. Implementation of EAE as tinnitus sound therapy
Thus, an appropriate EAE for tinnitus therapy would be
Where fm, τm, and Am are the frequency, delay, and amplitude of each tone, and
is the envelope of each pulse. Figure 1 shows the spectra of a pip tone, burst tone, and gammatone at the same frequency (f0= 500 Hz). A Blackman-Harris window has been used for the burst tone. For the pip tone the parameter pair (α,γ) = (3.225) is used. The three tones show a certain asymmetry in logarithmic scale. However, the gammatone has a slightly wider spectrum with cleaner left and right branches.
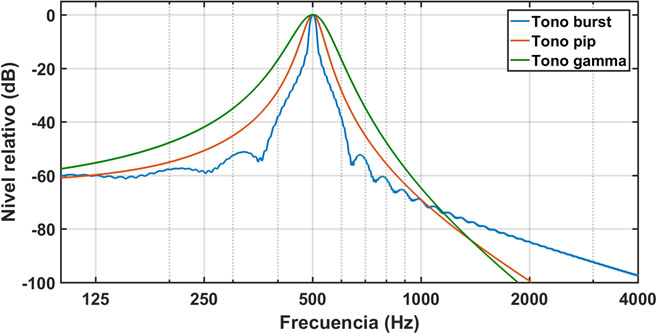
Figure 1: Spectra of a burst tone, a pip tone, and a gammatone, at the frequency of 500 Hz. A Blackman-Harris type window is used for the burst tone. A pair of parameters (α, γ) = (3.225) is used for the pip tone. The gamma tone is fully determined by the central frequency of 500 Hz. [Blue: Burst tone, Red: Pip Tone, Green: Gamma tone. X-axis: Frequency (Hz); Y-axis: Relative level (dB)]
Figure 2 shows the spectra of a sequence of gammatones at different frequencies. In this case a sequence of 10 seconds has been calculated at a rate of 4 pulses per second. The sequence therefore contains 40 gammatones. As can be seen, the amplitude of each tone is proportional to the hearing loss value at each frequency. A tinnitus patient with the HL in Figure 2, exposed to such a sequence, would perceive all tones at the same level (equalized amplitude sensation). Assuming that tinnitus is essentially triggered by the neural hearing system's attempt to compensate for the peripheral deprivation produced by HL, auditory training with this type of stimulus would work in the opposite direction, helping reduce the perception of tinnitus progressively and therefore, to their habituation.
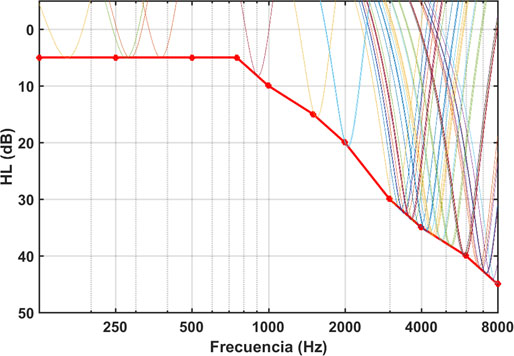
Figure 2: Spectra from a gamma sequence superimposed on the corresponding HL curve. [X-axis: Frequency (Hz), Y-axis: dB HL]
Cobo (2014) has developed a graphical user interface (GUI), Figure 3, for the design of an EAE therapy based on burst, pip, and gammatone sequences. Based on the patients' HL curves in each ear, the GUI allows the EAE to be generated as a diotic or dichotic sequence of tones, play it through the computer sound card or save it as an audio file for the patient to listen to through audio device, as directed by the specialist. The GUI also includes a signal properties section in which you can enter the sampling rate, in Hz, the signal length, in s, and the pulse rate per second. Another section draws the HL curves of each patient's ear, allowing better visual control of his/her hearing ability.
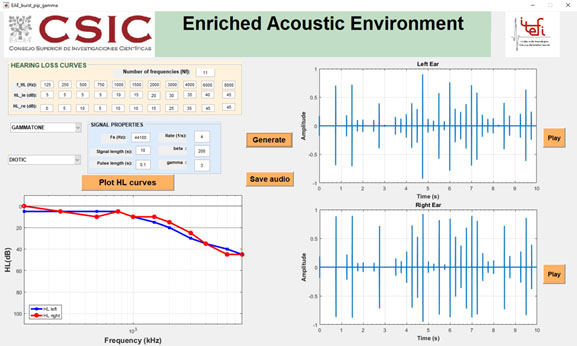
Figure 3: GUI for the design of an EAE for tinnitus sound therapy based on a sequence of tones.
Results
In 2018, a clinical study was initiated, with the approval of the Subcommittee on Bioethics of the CSIC, to test EAE therapy in tinnitus patients of varying aetiology. All participants in this study signed an informed consent form. Up to now, 140 patients have been recruited from whom 20 were excluded for different causes. The remaining 120 underwent a combined treatment of therapeutic counseling and sound therapy. The therapeutic counseling, which was applied in a single session of about 60 minutes at the start of treatment, consisted of a presentation explaining the mechanisms, epidemiology, and aetiology of tinnitus, as well as the foundations of EAE sound therapy. Personalized sound therapy consisted of an EAE based on a binaural sequence of burst, pip, or gammatones. Patients chose the most comfortable of the three tone types. The prescription was to hear this stimulus one hour a day for four months. 34 patients left treatment before completion and 11 others have not yet finished treatment. They were checked monthly through the Spanish version of the questionnaire Tinnitus Handicap Inventory (THI) (Herráiz et al., 2001). The THI is a 25-question questionnaire designed to measure the distress, handicap, and psychosocial consequences of tinnitus (Newman et al., 1998). According to the tinnitus gravity scale proposed by McCombe et al. (2001), 18 ≤ THI ≤ 36 would correspond to a mild tinnitus, 38 ≤ THI ≤ 56 to a moderate tinnitus, 58 ≤ THI ≤ 76 to a severe tinnitus, THI ≥ 78 to a catastrophic tinnitus.
The results in the 75 patients who have completed treatment are very promising and are under review in another scientific journal. Preliminary results in three patients undergoing a pip and gammatone EAE are presented here as an illustration.
Patient #1 is a 44-year-old male who was recruited for our study in January 2018. His tinnitus, located in the right ear, arose in November 2015, coinciding with a time of high stress. He previously had a stapedectomy of the right ear in 2012. His audiometry, figure 4A, showed normal hearing in the left ear and moderate losses at high frequencies in the right ear. He chose to be treated with a sequence of pip tones, with parameters (α, γ) = (3.200). After four months of treatment with this EAE, his THI was reduced from an initial value of 36 to a final value of 18, Figure 5.
Patient #2 is a 60-year-old male who was admitted to our study in July 2019. He had a tinnitus in his left ear for three years that arose after an episode of vertigo due to Ménière's disease. His audiometry, Figure 4B, shows normal hearing in the right ear, at low and medium frequencies, and a slight to moderate loss at high frequencies. In the left ear, however, he has moderate losses, at low frequencies, and profound losses at high frequencies. After following an EAE treatment with a gammatone sequence, his THI experienced a drop of 34 points, figure 5, from an initial value of 68 to a final value of 34.
Patient #3 is a 42-year-old woman who applied to join our study in March 2021. She had a tinnitus in her left ear for a year and a half. In December 2019 she was diagnosed with vestibular neuritis, which caused dizziness and tinnitus. Her audiometry, Figure 4C, shows normal hearing in the right ear with a slight scotoma at 4 kHz and light losses at high frequencies in the left ear. The severity of her tinnitus increased in November 2020, coinciding with her infection of COVID-19. She underwent a gammatone sequence-based EAE therapy between March and July 2021. Her THI fell by 40 points, from an initial value of 46 to a final value of 6 (Figure 5).
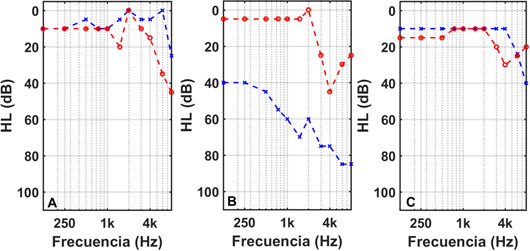
Figure 4: LH curves in the left (blue line) and right (red line) ears of the three patients undergoing tinnitus EAE therapy.
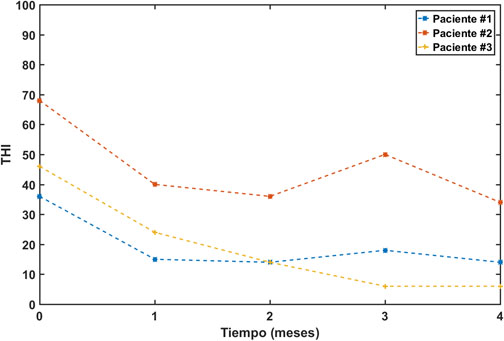
Figure 5: Evolution of THI in the three patients undergoing tinnitus EAE therapy. [Legend: Blue: Pacient #1, Red: Pacient #2, Pacient #3. X-axis: Time (months), Y-axis: THI.]
Discussion
EAE therapy, such as tinnitus retraining (TTR), is applied in combination with therapeutic counseling (Jastreff, 2015). The main difference between the two treatments is the type of stimulus used in sound therapy. The usual stimulus in TRT is a broadband noise, although other stimuli, such as coloured sounds (Henry et al., 2004) or filtered in a narrower band (Kim et al., 2014), and spectrally altered music (Li et al., 2016) have also been used. While broadband noise is valid for any patient type, the EAE stimulus is based on hearing loss, and is therefore customized for each subject. Although any type of sound is better than silence as tinnitus sound therapy, as long as it does not cause damage or molest (Jastreff & Jastreff, 2000), the EAE stimulus incorporates compensation for hearing loss, so it is more selective than wideband noise.
EAE therapy is implemented using a sequence of pip, burst or gammatones to be chosen by the patient. The pip and gammatone sequences were already used by other authors (Noreña and Eggermont, 2005; Noreña and Chery-Croze, 2007). Pip tones, which were used essentially in animal models, are more selective than gamma tones and have an envelope (and therefore a spectrum) that depends on two parameters (α,γ). These parameters take values that vary for each animal species tested. The direct application of these pip tones for tinnitus sound therapy therefore requires assigning the appropriate value to these parameters for the human species. The most novel contribution of this work is the incorporation of gammatones, in which the waveform (and spectrum) of each tone is determined exclusively by the frequency of the tone. Therefore, while the parameters of the pip tones are undefined, since they depend on the species tested, in the gammatones, they are perfectly defined.
The duration of the EAE treatment we are proposing (one hour a day, for four months) is much shorter compared to others found in the literature. Henry et al. (2006), Bauer et al. (2017) and Oishi et al. (2013), for example, presented the results of clinical trials using TRT for 18 to 24 months. However, using a personalized stimulus, such as EAE, a clinically relevant effect (reduction in THI greater than 20 points) can be achieved in a much shorter time.
This paper presents THI reduction results for three patients whose tinnitus were generated after an episode of stress (patient No.1), Ménière's disease (patient No.2), and vestibular neuritis (patient No.3). However, like TRT, EAE therapy can be applied to tinnitus patients of any aetiology (Jastreff, 2015). The only restrictions (exclusion criteria) are surgical operations (less than three months), recent episodes of vertigo (less than 15 days), or hydrocephalus. In addition, age less than 18 and greater than 75 years, and tinnitus severity (THI < 20) are considered to be exclusion criteria.
The tones in the EAE sequence include an amplitude factor that is proportional to the patient's hearing loss at each frequency. Also, because the stimulus is stereo, the different sensitivity of each ear is included. The result is a stereo stimulus that produces an equalized sensation in both ears at all frequencies. This equalized stimulus is appropriate for patients with mild to moderate losses and who do not have reduced sound tolerance (hyperacusis or misophonia). In patients with hyperacusis or misophonia, or with severe or profound hearing loss, care must be taken not to excessively amplify the pulses of the sequence. In these cases, the amplitude of the tones is adjusted as a compromise between the compensation of hearing loss and the threshold of sound intolerance.
Conclusions
The enriched acoustic environment consists of a random frequency tone sequence (pip, burst or gamma) within the hearing band and amplitude proportional to the hearing loss at that frequency. The spectrum of each gamma one in a frequency is a gamma filter that selectively stimulates the hearing system at that frequency. Therefore, a sequence of gammatones with amplitudes that compensate for the patient's hearing loss at each frequency is the optimal personalized sound stimulus for tinnitus sound therapy.
References
Baguley, D., McFerran, D, Hall, D. (2013). Tinnitus. Lancet, 382, 1600-1607. http://dx.doi.org/10.1016/S0140-6736(13)60142-7.
Bauer, C.A., Berry, J.L., Brozowski, T.J. (2017). The effect of Tinnitus Retraining Therapy on chronic tinnitus: A controlled trial. Laryngoscope Investig. Otolaryngol., 2, 166-177. doi: 10.1002/lio2.76.
Cobo, P. (2014). Ambiente Acústicamente Enriquecido para la terapia sonora del acúfeno. IX Congreso Iberoamericano de Acústica, FIA 2014, Valdivia (Chile).
Cuesta, M., Cobo, P. (2018). Relating tinnitus features and audiometric characteristics in a cohort of 34 tinnitus subjects. Loquens, 5, e054. https://doi.org/10.3989/loquens.2018.054
Eggermont, J.J. (2012). The Neuroscience of Tinnitus. Oxford University Press, Oxford (UK).
Henry, J.A., Rheinsburg, B., Zaugg, T. (2004). Comparison of custom sounds for achieving tinnitus relief. J. Am. Acad. Audiol., 15, 585-598. doi: 10.3342/ceo.2014.7.2.87.
Henry, J.A., Schechter, M.A., Zaugg, T.L., Griest, S., Jastreboff, P.J., Vernon, J.A., Kaelin, C., Meikle, M.B., Lyons, K.S., Stewards, B.J. (2006). Outcomes of clinical trial: Tinnitus Masking versus Tinnitus Retraining Therapy. J. Am. Acad. Audiol., 17, 104-132. doi: 10.3766/jaaa.17.2.4.
Henry, J.A. (2016). Measurement of tinnitus. Otol. Neurotol., 37, e276-e275. doi: 10.1097/MAO.0000000000001070.
Herráiz, C., Hernández Calvín, F.J., Plaza, G., Tapia, M.C., De los Santos, G. (2001). Evaluación de la incapacidad en los pacientes con acufenos. Acta Otorrinolaringol. Esp., 52, 142-145. doi: 10.1016/S0001-6519(01)78247-7.
Herráiz, C., Hernández Calvín, F.J. (2002). Acúfenos: Actualización. Ars Médica (Madrid).
Jastreboff, P.J. (1990). Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci. Res., 8, 221-254. doi: 10.1016/0168-0102(90)90031-9.
Jastreboff, P.J, Jastreboff, M.M. (2000). Tinnitus Retraining Therapy (TRT) as a method for treatment of tinnitus and hyperacusis patients. J. Am. Acad. Audiol., 11, 162-177.
Jastreboff, P.J. (2015). 25 years of tinnitus retraining therapy. HNO, 63, 307-311. doi: 10.1007/s00106-014-2979-1.
Kim, B.J., Chung, S.W., Jung, J.Y., Suh, M.W. (2014). Effect of different sounds on the treatment outcome of tinnitus retraining therapy. Clin. Exp. Otorhinolaryngol., 7, 87-93. doi: 10.3342/ceo.2014.7.2.87.
Li, S.A., Bao, L., Chrostowski, M. (2016). Investigating the effects of a personalized, spectrally altered music-based sound therapy on treating tinnitus: A blinded, randomized controlled trial. Audiol. Neurootol., 21, 296-304. doi: 10.1159/000450745.
López González, M.A. (2010). Factores etiopatogénicos de acúfenos. En Acúfeno como Señal de Malestar (López González y Esteban Ortega, Eds.), Capítulo 3, 22-30, CC2010, Sevilla.
McCombe, A., Baguley, D., Coles, R., McKeyna, L., McKinney, C., Windle-Taylor, P. (2001). Guidelines for the grading of tinnitus severty: the results of a working group commisioned by the British Association of Otolaryngologists. Clin. Otolaryngol. Allied Sci., 26, 388-93. doi: 10.1046/j.1365-2273.2001.00490.x.
Newman, C.W., Sandridge, S.A., Jacobson, G.P. (1998). Psychometric adequacy of the Tinnitus Handicap Inventory (THI) for evaluating treatment outcome. J. Am. Acad. Audiol., 9, 153-60.
Noreña, A.J., Eggermont, J.J. (2005). Enriched acoustic environment alter noise trauma reduces hearing loss and prevents cortical map reorganization. J. Neurosci., 25, 699-705. doi:10.1523/JNEUROSCI.2226-04.2005.
Noreña, A.J., Chery-Croze, S. (2007). Enriched acoustic environment rescales auditory sensitivity. NeuroReport, 18, 1251-1255. doi: 10.1097/WNR.0b013e3282202c35.
Oishi, N., Shinden, S., Kanzaki, S., Saito, K., Inoue, Y., Ogawa, K. (2013). Effects of tinnitus retraining therapy involving monaural noise generators. Eur. Arch. Otorhinolaryngol., 270, 443-448. doi: 10.1007/s00405-012-1951-5.
Patterson, R.D. (1994). The sound of a sinusoid: Spectral models. J. Acoust. Soc. Am., 96, 1409-1418. https://doi.org/10.1121/1.410285.
Paul, B.T., Bruce, I.C., Roberts, L.E. (2017). Evidence that hidden hearing loss underlies amplitude modulation encoding deficits in individuals with and without tinnitus. Hear. Res., 344, 170-182. https://doi.org/10.1016/j.heares.2018.04.005.
Pienkowski, M. (2019). Rationale and efficacy of sound therapies for tinnitus and hyperacusis. Neurosci., 407, 120-34. https://doi.org/10.1016/j.neuroscience.2018.09.012.
Schaette, R., Kempter, R. (2006). Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: a computational model. Eur. J. Neurosci., 23, 3124-3138. doi: 10.1111/j.1460-9568.2006.04774.x.
Tan, C.M., Lecluyse, W., McFerran, D., Meddis, R. (2013). Tinnitus and patterns of hearing loss. JARO, 14, 275-282. doi: 10.1007/s10162-013-0371-6.
Vio, M.M., Holmes, R.H. (2015). Hearing loss and tinnitus: 250 million people and a US$10 billion potential market. Drug Discovery Today, 10, 1263-1265. doi: 10.1016/S1359-6446(05)03594-4.
Conflict of interest
The author declares that he has no conflict of interest.
Funding
This research has not received external funding.
Acknowledgments
The participation of patients in this study is appreciated.
How to Cite:
Cobo, P. Preliminary results of the enriched acoustic environment as personalized sound-therapy for tinnitus. Auditio, 5(3).
License Type: Attribution 4.0 https://creativecommons.org/licenses/by/4.0/
Correspondence:
*Pedro Cobo
ITEFI (CSIC), Serrano 144, 28006 Madrid, Spain
Phone: +34 91 5618806
email: pedro.cobo@csic.es
Editorial Office
Production editor: Tomás Pérez Pazos
Translation: Louis M. de Ladebauche
Production: Publicaciones Académicas