Research Articles
Cochlear synaptopathy
1 Faculty of Medicine. Universitat de Vic - Universitat Central de Catalunya (UVic-UCC) / 2 Copenhagen Hearing and Balance Center (CHBC). Ear, Nose and Throat and Audiology clinic. Rigshospitalet University Hospital / 3 Hearing Systems section. Department of Health Technology. Technical University of Denmark (DTU).
 OPEN ACCESS
OPEN ACCESS
PEER REVIEWED
REVIEW ARTICLE
Abstract
Our understanding of cell structure damage in the peripheral auditory system due to acoustic overexposure and ageing underwent a paradigm shift with the discovery, over a decade ago, of cochlear synaptopathy (CS) – the permanent loss of synaptic connections between inner hair cells and auditory nerve fibres. Until then it was upheld that hair cells, and outer hair cells in particular, were the most vulnerable element in the peripheral auditory system. The classical paradigm of clinical audiological assessment has always been - and still is - based on measuring hearing thresholds with pure-tone audiometry. However, the discovery of CS has made it more urgent to develop new and more accurate diagnostic methods to detect hearing damage that is hidden in audiometry and to develop more specific tests for different types of peripheral cell damage. This article reviews the scientific literature on CS in animal models and discusses the evidence of CS in humans from cadaveric studies. Finally, after giving an overview of various inconclusive studies using psychoacoustic and physiological techniques in living humans, the article outlines some of the work currently underway in some European universities and future prospects for diagnosing and treating peripheral hearing loss.
Keywords
Hidden hearing loss, Audiology, auditory nerve, audiogram, synapse, evoked potentials, review article, computational models, Physiology
Clinical implications
Cochlear synaptopathy – the permanent loss of synaptic connections between inner hair cells and auditory nerve fibres – was demonstrated more than 10 years ago in animal models and, more recently, in human cadavers. Synaptic loss is undetectable in pure-tone audiometry, and yet it is highly likely to hinder acoustic signal perception in noisy environments. It is essential for audiology clinicians to be aware of the existence of cochlear synaptopathy and take patients seriously who, despite having normal audiometric thresholds, complain of hearing problems, with phrases like “I can hear but not understand”.
Received: 23.10.2023 Reviewed: 08.02.2024 Accepted: 09.04.2024 Published: 02.08.2024
Edited by:
Helia Relaño-Iborra
Universidad de Rochester, EE.UU.
Eriksholm Research Center, Denmark.
Reviewed by:
Carlos Gejo Linia
Universidad Católica San Antonio de Murcia, Murcia, España.
Enzo Aguilar-Vidal
Universidad de Chile, Santiago de Chile, Chile.
Joaquín Tomás Valderrama-Valenzuela
Universidad de Granada, Granada, España.
Macquarie University, Sydney, Australia.
Introduction
Pure-tone audiometry has been the gold standard audiological assessment for over seven decades, and its practical utility is universally acknowledged. However, we have known for years that the test is not sensitive to all peripheral auditory system conditions. Elevated hearing thresholds measured by tone audiometry is closely associated with outer hair cell (OHC) loss or dysfunction (Ryan and Dallos, 1975) and may also be associated with inner hair cell (IHC) dysfunction, but not IHC loss (Liberman and Kiang, 1984). In fact, audiometry is extremely insensitive to massive scattered IHC loss (Lobarinas et al., 2013) and also to auditory nerve (AN) fibre loss (Schuknecht and Woellner, 1955). In noise-induced hearing loss it was traditionally upheld that hair cells, and OHCs in particular, were the most vulnerable elements in the peripheral auditory system. Thus, hearing thresholds in the normal range (hearing level (HL) <20 dB at standard frequencies from 125 Hz to 8000 Hz) were understood to indicate a healthy auditory system, because OHCs (the most vulnerable elements) had to be healthy and functional to maintain those thresholds. However, in clinical practice, about 5% of patients complain of difficulties understanding speech, particularly in noisy environments, despite having normal hearing thresholds (<20 dB HL; Hind et al., 2011; Tremblay et al., 2015; Kumar et al., 2007; Saunders and Haggard, 1989; Cantuaria et al., 2021). This clinical evidence suggests the presence of some type of auditory pathway dysfunction, which often used to be attributed to central (i.e. brainstem) or cortical neural structures, and not to the periphery. This condition was given the name of hidden hearing loss (Schaette and McAlpine, 2011).
The aim of this paper is to review the most relevant scientific literature on hidden hearing loss and, more specifically, on cochlear synaptopathy (CS; see definition below). The review draws on the author's research experience in this field since 2014, but spans from the discovery of CS in 2009 in the mouse model (Kujawa and Liberman, 2009) to the present day. The paper focuses on the definition of CS based on animal model studies, on the development of diagnostic techniques, and on work undertaken to demonstrate and measure the presence of CS in living humans. Molecular aspects and structural changes in CS, and the development of imaging techniques are beyond the scope of this review. A longer version of this review can be found as a chapter in Manual de audiología laboral [Manual of occupational audiology] by Peñuela et al. (2022). A review of more recent, complementary literature in the field can be found in an article by Liu et al. (2024). The present review is structured as follows: the first part focuses on studies of noise-exposure CS in animal models, and the effects of ageing on synapse loss in the AN, also in animal models. It continues with an overview of the different studies on hidden hearing loss in humans, noting the variability and divergence of results, which are inconclusive. The last part looks into the future and at prospective diagnostic and pharmacological techniques for managing CS in humans.
Noise-induced cochlear synaptopathy
Cochlear synaptopathy is described as a permanent disconnection, interruption or loss of the synapses connecting with the IHCs in the cochlea (Kujawa and Liberman, 2009). The first report of CS was from a study on noise-exposed mice subjected to 105 dB sound pressure level (SPL) for two hours, producing an immediate temporary elevation of hearing thresholds of about 30-40 dB (measured by distortion product otoacoustic testing [DPOAE] and auditory brainstem responses [ABR]), which returned to pre-exposure values about two weeks post-exposure (Figure 1H and I). Using imaging techniques, the authors viewed different cochlear regions and counted the IHCs, AN fibres and synaptic receptors paired to synaptic ribbons in the IHCs (Figure 1A and B). Each peripheral axon of the AN (green filaments, Figure 1A and B) makes a single synaptic connection with a synaptic ribbon (red dots, Figure 1A and B) in one IHC (IHC nucleus in blue, Figure 1A and B). Synapse losses of 50-55% were found in the basal region of noise-exposed mice compared with the control mice. While the DPOAE and ABR-wave-I measured thresholds returned to pre-exposure values (Figure 1H and I), synaptic losses were permanent (i.e., no synapse recovery was found over time). In addition, no evidence of IHC or OHC loss was found in any cochlear region. In this respect, no differences were found between exposed and control animals in DPOAE amplitude measurements by stimulation level (Figure 1H), since the OHCs were intact in the exposed animals. However, noise-exposed animals did show permanent functional effects in the form of a permanent reduction of the supra-threshold levels of ABR wave I (Figure 1I). Later studies have demonstrated a high correlation between ABR wave I amplitude and cochlear synapse survival (Sergeyenko et al., 2013; Parthasarathy and Kujawa, 2018). The presence of CS has been demonstrated in other mammals such as guinea pigs (Lin et al., 2011; Liu et al., 2012), rats (Lobarinas et al., 2017), chinchillas (Hickman et al., 2018; Hickox et al., 2017), Rhesus macaques (Valero et al., 2017) and humans (Makary et al., 2011; Viana et al., 2015; Wu et al., 2019).
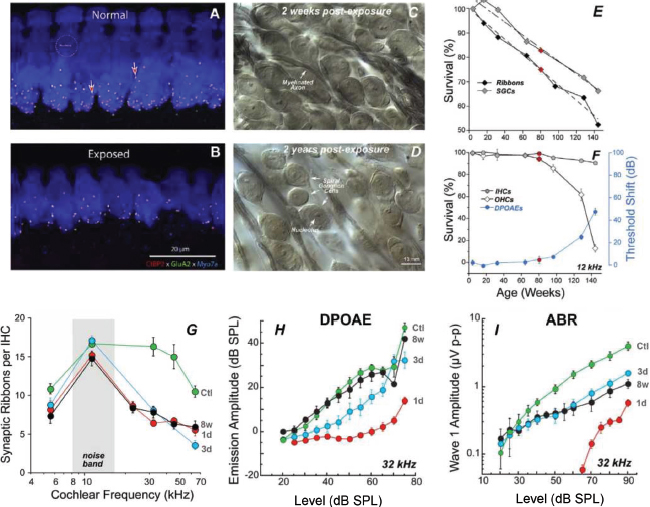
Figure 1. Panels A and B show synapse loss in immunostained confocal images of a control (A) and a noise-exposed mouse (B). Various markers identify the different structures: CtBP2 (red) identifies the presynaptic ribbons, GluA2 (green) the postsynaptic receptors, and Myosin VIIa (blue) the hair cells. Panels C and D show the degradation of spiral ganglion cells in noise-exposed mice in osmium-stained cochlear sections two weeks (C) and two years (D) post-exposure (Liberman and Kujawa, 2017). Panel E shows presynaptic ribbon and spiral ganglion cell survival, by age. Panel F shows IHC and OHC survival, by age, and association with DPOAEs (Sergeyenko et al., 2013). Panel G shows synapse loss count at different post-exposure times (1d: 1 day, 3d: 3 days and 8w: 8 weeks) versus control mice (Ctl: control). Panels H and I show the effect of synapse loss in DPOAEs and ABRs, respectively, by stimulation level. In DPOAEs and ABRs alike, thresholds are recovered by 8 weeks. However, at supra-threshold levels, DPOAE amplitudes are fully recovered but ABRs show reduced amplitude at high levels (Kujawa and Liberman, 2009).
Figures reproduced by permission of the authors (Kujawa and Liberman, 2009; Liberman and Kujawa, 2017; Sergeyenko et al., 2013) and the publisher (Copyright 2009, Society for Neuroscience, under a CC-BY-NC-SA licence).
After the initial discovery of CS, subsequent studies found that not all AN fibres were equally affected. A study by Furman et al. (2013) suggested that synapse loss was much more selective for fibres with low- and medium-spontaneous rates (SR), hardly affecting those with high-SR. In the AN, two to three subgroups of afferent neurons can be identified according to their spontaneous discharge rate (i.e., the number of action potentials generated in the absence of stimulation). In cats, which are sensitive to low and medium frequency ranges, three types of neurons have been demonstrated: high-SR neurons (more than 18 discharges/second), medium-SR neurons (between 0.5 and 18 discharges/second) and low-SR neurons (less than 0.5 discharges/second); Liberman, 1978). In other mammals sensitive to higher frequency ranges, such as mice, two types of neurons have been described: high-SR (>1 discharge/second) and low-SR (<1 discharge/second); Taberner and Liberman, 2005). It is thought that the characteristics of the human AN resembles the cat AN more than the mouse AN, but this is still to be demonstrated. Neuronal spontaneous discharge rate is associated with the neuronal excitation threshold. High-SR neurons are sensitive to low thresholds, while low-SR neurons are sensitive to high thresholds (Liberman, 1978). In addition to these functional differences, there are also morphological differences. The same IHC receives synaptic connections from all three types of AN neurons (high-, medium- and low-SR neurons). However, low-SR neurons tend to innervate the modiolar side of the IHC, whereas high-SR neurons tend to innervate the pillar side. In addition, low-SR fibres tend to have thinner axons and fewer mitochondria, whereas high-SR fibres have thicker axons and more mitochondria (Liberman, 1982). The study by Furman et al. (2013) used these modiolar/pillar gradients in AN synapse innervation (i.e., the IHC side on which fibres are innervated) in exposed animals versus controls. They concluded that there was more loss of low- and medium-SR fibres in exposed animals. This finding was also corroborated by direct measurements of individual fibres that showed differences in the statistical distributions of spontaneous discharge rate between the control and exposed animals.
The study by Furman et al. (2013), which found that CS was selective for low- and medium-SR fibres, had a major impact on the design of both electrophysiological and psychoacoustic experiments in humans. This also explained why hearing thresholds were preserved in CS, and why, on the contrary, supra-threshold responses (i.e. high-level ABR wave I amplitude) were indeed affected. If CS did not affect high-SR and low threshold fibres, the fibres could still encode the low intensity signals used in threshold measurements; at the same time, the selective loss of low-SR and high-threshold neurons would lead to problems encoding the acoustic signal at supra-threshold levels. However, some authors began to question these results and proposed that CS affected all AN fibres, regardless of spontaneous discharge rates. Indeed, a reanalysis of the data by Furman et al. (2013) found that in the same original study there was actually a loss of high-SR fibres of more than 26%, which had not been clearly reported (Marmel et al., 2015). In addition, most studies that used computational models to predict the effect of CS have been forced to apply significant loss affecting high-SR fibres (Paul et al., 2017; Verhulst et al., 2018; Encina-Llamas et al., 2019; Keshishzadeh et al., 2020, 2021; Johannesen et al., 2022). A more recent study conducted in mice has provided evidence contrary to what was suggested by Furman et al.(2013), finding that CS was not selective for low- and medium-SR fibres (Suthakar and Liberman, 2021). The study measured the direct response of single fibres in control mice and in noise-exposed mice sustaining more than 50% loss with CS. Measurements in surviving AN fibres in the noise-exposed mice showed no difference in the statistical distributions of spontaneous discharge rate or in neuronal properties versus measurements in the control mice, suggesting that all three fibre types sustained similar losses from sound exposure. It is interesting that this 2021 study was conducted in mice, like the original CS study by Kujawa and Liberman (2009), whereas the study by Furman et al. (2013) was done in guinea pigs. Recently, it has been shown that over time, guinea pigs are able to regenerate synapses lost immediately after noise exposure (Hickman et al., 2020, 2021; Shi et al., 2013), which may explain the discrepancy between studies in mice and guinea pigs and computational models. In other species such as chinchillas, no evidence of synaptic regeneration has been observed (Bharadwaj et al., 2022). In humans, studies in temporal bones obtained at autopsy show clear synaptic loss with age, with a fibre degeneration rate similar to that in mice (Wu et al., 2019, 2020), suggesting an absence of cochlear synaptic regeneration in humans.
Age-related cochlear synaptopathy
As part of the ageing process, the number of active synaptic connections between the AN and the IHCs declines naturally. In healthy ageing animals, synaptic loss has been shown to occur steadily and continuously over the animal’s lifespan, reaching a 50% loss in the oldest specimens (Sergeyenko et al., 2013). As in the case of noise-induced CS, age-related CS precedes hair cell loss and threshold elevation (Figure 1F), which are minimal until advanced age. Synaptic loss is followed by the corresponding spiral ganglion cell loss, but with a time lag (Figure 1E) that is very similar to the degeneration of the entire AN fibre also found in human temporal bones (Makary et al., 2011). Noise exposure accelerates this natural age-related synaptic loss (Fernandez et al., 2015). The functional effects of age-related CS are similar to those of noise-induced CS: DPOAEs remain unchanged providing there is no OHC loss, the ABR wave I amplitude is reduced as the individual ages, in clear correlation with the synapse count (Sergeyenko et al., 2013), and the amplitude of steady-state evoked potentials such as envelope-following responses (EFR) decreases with age in correlation with the synapse count (Parthasarathy and Kujawa, 2018).
Cochlear synaptopathy in humans
The existence of cochlear synaptopathy in humans has been the subject of much debate among the scientific community in the past (Bramhall et al., 2019), and was finally demonstrated in human histopathological studies on temporal bones obtained at autopsy (Wu et al., 2019, 2021). These studies showed a clear age-dependent loss of synapses in humans, similar to that in animals, and occurring at a higher rate than in prior hair cell loss. However, attempts to find evidence of CS in living humans have been much more controversial (Valderrama et al., 2022), because studies in living humans have several additional difficulties compared to studies in animals: 1) human genetics shows much greater diversity than some rodents, especially mice, which are almost genetic copies of each other. Genetic diversity causes greater variability in the individual effects of an auditory system insult, such as noise exposure, and greater variability in potential biomarkers; 2) in animal studies, researchers have worked long and hard to find a noise intensity and exposure time that causes significant synapse loss without hair cell loss. It is therefore possible to study CS in laboratory animals in complete isolation from other pathologies, whereas in humans a controlled environment is almost impossible, and various pathologies (e.g., CS, OHC and IHC loss and/or dysfunction, stria vascularis degeneration and spiral ganglion cell loss) are inevitably present concomitantly in the same individual; 3) in living humans it is ethically impossible to perform a histopathological study to count cochlear synapses. In short, real evidence is impossible in living humans.
Researchers have tackled these problems using four main strategies:
A) They have assessed lifetime noise exposure through questionnaires, relating it to one or more physiological biomarkers sensitive to CS in animals. Most of these studies used ABR wave I amplitude as a biomarker of CS and did not find clear and significant correlations between wave I amplitude and sound exposure estimation (Prendergast et al., 2017a; Stamper and Johnson, 2015; Fulbright et al., 2017; Spankovich et al., 2017; Grinn et al., 2017; Ridley et al., 2018; Maele et al., 2021). Other studies have used dosimeters to measure sound exposure, with the disadvantage that sound exposure estimation is obviously limited by time. These studies either found no effect or only small effects on ABR latency (Skoe and Tufts, 2018; Maele et al., 2021). Other studies used EFR magnitude as a biomarker without finding any association (Prendergast et al., 2017a; Guest et al., 2017b,a; Grose et al., 2017). However, some studies have found some association between noise exposure and a CS-related physiological response, but mostly showed weak effects. For example, effects on wave I amplitude have been reported (Valderrama et al., 2018; Bramhall et al., 2018a) on the relationship between the summating potential (SP) and the action potential (AP; equivalent to the ABR wave I) (Liberman et al., 2016; Grose et al., 2017), and on EFR magnitude (Bharadwaj et al., 2015; Bramhall et al., 2021).
B) The second strategy was also based on assessing patients’ lifetime noise exposure through questionnaires and relating it to one or more CS-associated speech perception measures by means of heuristic argumentation, i.e., following an undemonstrated logical composition of arguments, such as that CS degrades speech intelligibility in noise (Lopez-Poveda and Barrios, 2013; Lopez-Poveda, 2014). Most of these studies have shown no clear, significant correlation between sound exposure estimation and various behavioural hearing tests presumed to be affected by cochlear synapse loss (Prendergast et al., 2017b; Yeend et al., 2017; Prell et al., 2018; Fulbright et al., 2017; Grinn et al., 2017; Maele et al., 2021; Grose et al., 2017; Guest et al., 2018). One study did show worse speech-in-noise intelligibility among young music students assigned to the high-risk group based on self-report for noise exposure (Liberman et al., 2016).
C) The aim of the third strategy was to find correlations between various physiological and behavioural measurements thought to be sensitive to CS, derived from animal studies. Again, a number of studies showed no significant correlations between wave I amplitude and speech-in-noise intelligibility (Fulbright et al., 2017; Grinn et al., 2017; Maele et al., 2021; Prendergast et al., 2017b; Guest et al., 2018; Bramhall et al., 2018a; Johannesen et al., 2019), EFR magnitude (Maele et al., 2021; Prendergast et al., 2017b; Guest et al., 2018) or the relationship between ABR I- and V-wave amplitudes and speech-in-noise intelligibility(Guest et al., 2018). However, one study did report significant correlation with speech-in-noise intelligibility. For example, an association was found between SP and AP (or wave I amplitude) and speech-in-noise intelligibility (Liberman et al., 2016; Grant et al., 2020), although the reliability of this measure has been found to lack robustness (Prendergast et al., 2018). Speech intelligibility has also been associated with EFR magnitude (Mepani et al., 2021). Another study showed worsening of speech intelligibility in subjects who had longer interpeak latencies for ABR waves I to V (an indicator of neural transmission time between the AN and inferior colliculus). The same subjects also had lower wave I to V amplitude ratios (indicative of elevated central gain, see below); Valderrama et al., 2018). Finally, a correlation was reported between the wave-V latency of ABRs measured in masking noise and the detection of interaural timing difference in the envelope of the acoustic stimuli (Mehraei et al., 2016).
D) The fourth and last strategy was to find a relationship between the existence of CS, based on measurements derived from animal studies, and the presence of tinnitus. Different studies have shown that, despite a reduction in ABR wave I amplitudes in normal ears presumably related to the existence of CS, wave-V amplitude remain unchanged (Burkard and Sims, 2001; Johannesen et al., 2019; Grose et al., 2019; Rumschlag et al., 2022; Johannesen and Lopez-Poveda, 2021; Schaette and McAlpine, 2011; Temboury-Gutierrez et al., 2024b). This phenomenon has been related to the concept of central gain, which would explain overexcitation of the central auditory system (i.e., the brainstem) as a result of reduced central neuronal inhibition (Heeringa and van Dijk, 2014) compensating the reduced peripheral activity due to synapse loss (Chambers et al., 2016; Auerbach et al., 2014; Sheppard et al., 2018; Mohrle et al., 2019; Johannesen and Lopez-Poveda, 2021; Salvi et al., 2017; Caspary et al., 2008; Lai et al., 2017; Parthasarathy et al., 2019; Diehl and Schaette, 2015), which would also cause heightened cortical activity (Zan et al., 2020). One current hypothesis links central overexcitation to the presence of tinnitus; i.e, the perception of sound in the absence of a real external sound source (Mohrle et al., 2016; Eggermont, 2017; Knipper et al., 2013; Schaette, 2014; Schaette and McAlpine, 2011). Some CS biomarkers have also been associated with tinnitus, such as the acoustic reflex (Wojtczak et al., 2017), but a later study found no such link (Guest et al., 2019). Reduced EFR magnitude has also been associated with tinnitus (Paul et al., 2017), but a review of the same study ultimately found that the effect was not statistically significant (Roberts et al., 2018).
In summary, CS studies in living humans give contradictory and therefore inconclusive results. This status quo calls for new, more imaginative studies combining different techniques and tests, making use of the latest technology and computing power.
Prospective treatments and diagnostic techniques
Today’s treatments are unable to completely reverse hearing loss, although solutions exist to compensate or alleviate the effects of hearing loss through auditory rehabilitation using hearing aids or cochlear implants. However, there is speculation that in the coming decades, pharmacological solutions may reverse or prevent some hearing impairments. These speculations need three things to happen at the same time to become a reality: 1) specific and efficient drugs must be developed to restore damaged cells safely and without side effects, backed by the corresponding clinical trials; 2) surgical techniques need to be developed to deliver drugs in an efficient and controlled way to the damaged cochlear sections; and 3) accurate diagnostic techniques need to be developed to assess the degree of degeneration and damage to the different cell types in the peripheral auditory system in individual patients. In recent years, various research projects on CS have focused on the use of neurotrophic factors in neuronal regeneration (Cassinotti et al., 2022; Suzuki et al., 2016; Foster et al., 2022b; Leake et al., 2020; Hashimoto et al., 2019), some of which have reached different stages in clinical trials (Foster et al., 2022a). The aim of these techniques is to regenerate synapses on AN fibres that have been disconnected, and achieve reconnection to the corresponding IHC. Some authors, albeit fewer, have reported on transtympanic techniques and strategies to deliver drugs locally to the cochlea (Maxwell et al., 2021; Foster et al., 2022a). In addition, some surgeons at Rigshospitalet hospital in Copenhagen, Denmark, and others elsewhere, are developing intracochlear drug delivery surgical techniques in conjunction with other researchers in the Danish audiology sector (author's private sources).
Some researchers are making remarkable progress in individualised, targeted diagnostics, even though no techniques currently exist to clinically assess CS. As mentioned earlier, CS in humans is most likely to occur together with other types of cochlear cell loss or dysfunction. Solutions are therefore likely to need a combination of different experimental measurements, probably assisted by computational modelling, together with efficient use of artificial intelligence (AI) models. Along these lines, various studies have used computational models to predict the effect of CS and other hearing losses on different physiological responses (Paul et al., 2017; Keshishzadeh et al., 2020; Verhulst et al., 2018; Encina-Llamas et al., 2019, 2021; Märcher-Rørsted et al., 2022). Recently, researchers conducting studies at Ghent University in Belgium have developed a framework that combines computational physiological models with AI models to develop new auditory processing strategies to compensate some auditory pathologies, such as CS and OHC loss (Bramhall et al., 2018b; Buran et al., 2022; Drakopoulos et al., 2021, 2022; Drakopoulos and Verhulst, 2023; Drakopoulos et al., 2023). Briefly, good computational physiological models exist that predict the AN response to any acoustic stimulus (Bruce et al., 2018; Verhulst et al., 2018) and AI models (neural networks) can be trained to produce responses that are almost identical to the physiological model. These researchers built two AI models: a healthy model to simulate a normal-hearing AN response and a hearing-impaired one with a hearing impairment simulating a damaged response. Equipped with these two responses, they are then able to build a third AI model coupled to the input of the hearing-impaired model with the aim of reducing the difference between the hearing-impaired response and the normal-hearing response (see Figure 1 in Drakopoulos and Verhulst, 2023), so that the hearing-impaired model response resembles the normal-hearing model response (to compensate the loss). The result lends itself to ideal hearing aid processing. The authors noted that OHC losses are more easily compensated than synapse losses.
Concurrently, research groups that the present author is affiliated with at the Technical University of Denmark (DTU), together with the Copenhagen Hearing and Balance Center (CHBC) at Rigshospitalet hospital in Copenhagen, have been refining the framework model developed by Dau (2003) to simulate cochlear electrophysiological responses (through electrocochleography, ECochG) in both healthy and various peripheral hearing-loss profiles (Temboury-Gutierrez et al., 2024a). The aim of this research is to develop an AI model based on one or more ECochG responses in an individual patient that can predict which combination of CS, IHC dysfunction and OHC loss/dysfunction is most likely to be present. To do this, it is essential to develop computational models that accurately predict the AN response in humans. Based on these investigations, evoked potentials such as frequency-following responses (FFR) may potentially be a sensitive biomarker of CS and also robust to other losses, such as OHC loss or dysfunction, as corroborated in computational models (Märcher-Rørsted et al., 2022; Temboury-Gutierrez et al., 2024b). Similar studies in the chinchilla animal model appear to support these results in humans. An alternative type of evoked potential are EFRs (Encina-Llamas et al., 2019; Keshishzadeh et al., 2020, 2021; Vasilkov et al., 2021), which were shown to be sensitive to CS in mice (Parthasarathy and Kujawa, 2018). In short, it appears that a combination of steady-state evoked potentials with different types of advanced computational models may be the key to making accurate hearing diagnoses in humans. When these new methods have been demonstrated and replicated, we will then need to find ways to adapt them to the needs of a clinical practice setting.
Conclusion
The classical paradigm regarding damage from acoustic overexposure and ageing upheld that OHCs were the most vulnerable element in the peripheral auditory system. In 2009, it was demonstrated in mice models that CS – the permanent loss of synaptic connections between IHCs and AN fibres – preceded hair cell loss. This synaptic loss does not affect hearing thresholds and is therefore hidden on pure-tone audiometry. However, it does cause a reduction in the supra-threshold response in the AN and is likely to hinder sound perception in noisy environments. Cochlear synaptopathy has been demonstrated in several mammals, including humans. It occurs naturally as part of the ageing process and is exacerbated by sound overexposure. Studies in humans using psychoacoustic measurements lack full consensus in their results. More recently, some steady-state evoked potentials such as EFRs and FFRs have shown more potential to be considered as good biomarkers sensitive to CS. It is anticipated that in the next few years, these evoked potentials will be combined with physiological computational models and AI models to help diagnose CS in humans accurately and reliably.
References
Auerbach, B. D., Rodrigues, P. V., & Salvi, R. J. (2014). Central gain control in tinnitus and hyperacusis. Frontiers in Neurology, 5, 1-21, https://doi.org/10.3389/fneur.2014.00206.
Bharadwaj, H. M., Hustedt-Mai, A. R., Ginsberg, H. M., Dougherty, K. M., Muthaiah, V. P. K., Hagedorn, A., Simpson, J. M., & Heinz, M. G. (2022). Cross-species experiments reveal widespread cochlear neural damage in normal hearing. Communications Biology, 5, 733, https://doi.org/10.1038/s42003-022-03691-4.
Bharadwaj, H. M., Masud, S., Mehraei, G., Verhulst, S., & Shinn-Cunningham, B. G. (2015). Individual differences reveal correlates of hidden hearing deficits. The Journal of Neuroscience, 35, 2161-2172, https://doi.org/10.1523/JNEUROSCI.3915-14.2015.
Bramhall, N., Beach, E. F., Epp, B., Le Prell, C. G., Lopez-Poveda, E. A., Plack, C. J., Schaette, R., Verhulst, S., & Canlon, B. (2019). The search for noise-induced cochlear synaptopathy in humans: Mission impossible? Hearing Research, 377, 88-103, https://doi.org/10.1016/j.heares.2019.02.016.
Bramhall, N. F., Konrad-Martin, D., & McMillan, G. P. (2018a). Tinnitus and auditory perception after a history of noise exposure: Relationship to auditory brainstem response measures. Ear Hearing, 39, 881-894, https://doi.org/10.1097/AUD.0000000000000544.
Bramhall, N. F., McMillan, G. P., & Kampel, S. D. (2021). Envelope following response measurements in young veterans are consistent with noise-induced cochlear synaptopathy. Hearing Research, 408, 108310, https://doi.org/10.1016/j.heares.2021.108310.
Bramhall, N. F., McMillan, G. P., Kujawa, S. G., & Konrad-Martin, D. (2018b). Use of non-invasive measures to predict cochlear synapse counts. Hearing Research, 370, 113-119, https://doi.org/10.1016/j.heares.2018.10.006.
Bruce, I. C., Erfani, Y., & Zilany, M. S. (2018). A phenomenological model of the synapse between the inner hair cell and auditory nerve: Implications of limited neurotransmitter release sites. Hearing Research, 360, 40-54, https://doi.org/10.1016/j.heares.2017.12.016.
Buran, B. N., McMillan, G. P., Keshishzadeh, S., Verhulst, S., & Bramhall, N. F. (2022). Predicting synapse counts in living humans by combining computational models with auditory physiology. The Journal of the Acoustical Society of America, 151, 561-576, https://doi.org/10.1121/10.0009238.
Burkard, R. F. & Sims, D. (2001). The human auditory brainstem response to high click rates. American Journal of Audiology, 10, 53-61, https://doi.org/10.1044/1059-0889(2001/008).
Cantuaria, M. L., Pedersen, E. R., Waldorff, F. B., Sørensen, M., & Schmidt, J. H. (2021). Hearing examinations in southern denmark (hesd) database: a valuable tool for hearing-related epidemiological research. International Journal of Audiology, 60, 300-311, https://doi.org/10.1080/14992027.2020.1831702.
Caspary, D. M., Ling, L., Turner, J. G., & Hughes, L. F. (2008). Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. Journal of Experimental Biology, 211, 1781-1791, https://doi.org/10.1242/jeb.013581.
Cassinotti, L. R., Ji, L., Borges, B. C., Cass, N. D., Desai, A. S., Kohrman, D. C., Liberman, M. C., & Corfas, G. (2022). Cochlear neurotrophin-3 overexpression at mid-life prevents age-related inner hair cell synaptopathy and slows age-related hearing loss. Aging Cell, 21, https://doi.org/10.1111/acel.13708.
Chambers, A. R., Resnik, J., Yuan, Y., Whitton, J. P., Edge, A. S., Liberman, M. C., & Polley, D. B. (2016). Central gain restores auditory processing following near-complete cochlear denervation. Neuron, 89, 867-879, https://doi.org/10.1016/j.neuron.2015.12.041.
Dau, T. (2003). The importance of cochlear processing for the formation of auditory brainstem and frequency following responses. The Journal of the Acoustical Society of America, 113, 936-950, https://doi.org/10.1121/1.1534833.
Diehl, P. U. & Schaette, R. (2015). Abnormal auditory gain in hyperacusis: Investigation with a computational model. Frontiers in Neurology, 6, 15, https://doi.org/10.3389/fneur.2015.00157.
Drakopoulos, F., Baby, D., & Verhulst, S. (2021). A convolutional neural-network framework for modelling auditory sensory cells and synapses. Communications Biology, 4, 827, https://doi.org/10.1038/s42003-021-02341-5.
Drakopoulos, F., Broucke, A. V. D., & Verhulst, S. (2023). A dnn-based hearing-aid strategy for real-time processing: One size fits all. IEEE https://ieeexplore.ieee.org/document/10094887/.
Drakopoulos, F., Vasilkov, V., Vecchi, A. O., Wartenberg, T., & Verhulst, S. (2022). Model-based hearing-enhancement strategies for cochlear synaptopathy pathologies. Hearing Research, 424, 108569, https://doi.org/10.1016/j.heares.2022.108569.
Drakopoulos, F. & Verhulst, S. (2023). A neural-network framework for the design of individualised hearing-loss compensation. IEEE/ACM Transactions on Audio, Speech, and Language Processing, 31, 2395-2409, https://doi.org/10.1109/TASLP.2023.3282093.
Eggermont, J. J. (2017). Effects of long-term non-traumatic noise exposure on the adult central auditory system. hearing problems without hearing loss. Hearing Research, 352, 12-22, https://doi.org/10.1016/j.heares.2016.10.015.
Encina-Llamas, G., Dau, T., & Epp, B. (2021). On the use of envelope following responses to estimate peripheral level compression in the auditory system. Scientific Reports, 11, 6962, https://doi.org/10.1038/s41598-021-85850-x.
Encina-Llamas, G., Harte, J. M., Dau, T., Shinn-Cunningham, B., & Epp, B. (2019). Investigating the effect of cochlear synaptopathy on envelope following responses using a model of the auditory nerve. Journal of the Association for Research in Otolaryngology, 20, 363-382, https://doi.org/10.1007/s10162-019-00721-7.
Fernandez, K. A., Jeffers, P. W., Lall, K., Liberman, M. C., & Kujawa, S. G. (2015). Aging after noise exposure: Acceleration of cochlear synaptopathy in “recovered” ears. The Journal of Neuroscience, 35, 7509-7520, https://doi.org/10.1523/JNEUROSCI.5138-14.2015.
Foster, A. C., Jacques, B. E., & Piu, F. (2022a). Hearing loss: The final frontier of pharmacology. Pharmacology Research Perspectives, 10, e00970, https://doi.org/10.1002/prp2.970.
Foster, A. C., Szobota, S., Piu, F., Jacques, B. E., Moore, D. R., Sanchez, V. A., & Anderson, J. J. (2022b). A neurotrophic approach to treating hearing loss: Translation from animal models to clinical proof-of-concept. The Journal of the Acoustical Society of America, 151, 3937-3946, https://doi.org/10.1121/10.0011510.
Fulbright, A., Prell, C. L., Griffiths, S., & Lobarinas, E. (2017). Effects of recreational noise on threshold and suprathreshold measures of auditory function. Seminars in Hearing, 38, 298-318, https://doi.org/10.1055/s-0037-1606325.
Furman, A. C., Kujawa, S. G., & Liberman, M. C. (2013). Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. Journal of Neurophysiology, 110, 577-586, https://doi.org/10.1152/jn.00164.2013.
Grant, K. J., Mepani, A. M., Wu, P., Hancock, K. E., de Gruttola, V., Liberman, M. C., & Maison, S. F. (2020). Electrophysiological markers of cochlear function correlate with hearing-in-noise performance among audiometrically normal subjects. Journal of Neurophysiology, 124, 418-431, https://doi.org/10.1152/jn.00016.2020.
Grinn, S. K., Wiseman, K. B., Baker, J. A., & Prell, C. G. L. (2017). Hidden hearing loss? no effect of common recreational noise exposure on cochlear nerve response amplitude in humans. Frontiers in Neuroscience, 11, https://doi.org/10.3389/fnins.2017.00465.
Grose, J. H., Buss, E., & Elmore, H. (2019). Age-related changes in the auditory brainstem response and suprathreshold processing of temporal and spectral modulation. Trends in Hearing, 23, 233121651983961, https://doi.org/10.1177/2331216519839615.
Grose, J. H., Buss, E., & Hall, J. W. (2017). Loud music exposure and cochlear synaptopathy in young adults: Isolated auditory brainstem response effects but no perceptual consequences. Trends in Hearing, 21, 233121651773741, https://doi.org/10.1177/2331216517737417.
Guest, H., Munro, K. J., & Plack, C. J. (2017a). Tinnitus with a normal audiogram: Role of high-frequency sensitivity and reanalysis of brainstem-response measures to avoid audiometric over-matching. Hearing Research, 356, 116-117, https://doi.org/10.1016/j.heares.2017.10.002.
Guest, H., Munro, K. J., Prendergast, G., Howe, S., & Plack, C. J. (2017b). Tinnitus with a normal audiogram: Relation to noise exposure but no evidence for cochlear synaptopathy. Hearing Research, 344, 265-274, https://doi.org/10.1016/j.heares.2016.12.002.
Guest, H., Munro, K. J., Prendergast, G., Millman, R. E., & Plack, C. J. (2018). Impaired speech perception in noise with a normal audiogram: No evidence for cochlear synaptopathy and no relation to lifetime noise exposure. Hearing Research, 364, 142—151, https://doi.org/10.1016/j.heares.2018.03.008.
Guest, H., Munro, K. J., Prendergast, G., & Plack, C. J. (2019). Reliability and interrelations of seven proxy measures of cochlear synaptopathy. Hearing Research, 375, 34-43, https://doi.org/10.1016/j.heares.2019.01.018.
Hashimoto, K., Hickman, T. T., Suzuki, J., Ji, L., Kohrman, D. C., Corfas, G., & Liberman, M. C. (2019). Protection from noise-induced cochlear synaptopathy by virally mediated overexpression of nt3. Scientific Reports, 9, 15362, https://doi.org/10.1038/s41598-019-51724-6.
Heeringa, A. & van Dijk, P. (2014). The dissimilar time course of temporary threshold shifts and reduction of inhibition in the inferior colliculus following intense sound exposure. Hearing Research, 312, 38-47, https://doi.org/10.1016/j.heares.2014.03.004.
Hickman, T. T., Hashimoto, K., Liberman, L. D., & Liberman, M. C. (2020). Synaptic migration and reorganization after noise exposure suggests regeneration in a mature mammalian cochlea. Scientific Reports, 10, 19945, https://doi.org/10.1038/s41598-020-76553-w.
Hickman, T. T., Hashimoto, K., Liberman, L. D., & Liberman, M. C. (2021). Cochlear synaptic degeneration and regeneration after noise: Effects of age and neuronal subgroup. Frontiers in Cellular Neuroscience, 15, https://doi.org/10.3389/fncel.2021.684706.
Hickman, T. T., Smalt, C., Bobrow, J., Quatieri, T., & Liberman, M. C. (2018). Blast-induced cochlear synaptopathy in chinchillas. Scientific Reports, 8, 10740, https://doi.org/10.1038/s41598-018-28924-7.
Hickox, A. E., Larsen, E., Heinz, M. G., Shinobu, L., & Whitton, J. P. (2017). Translational issues in cochlear synaptopathy. Hearing Research, 349, 164-171, https://doi.org/10.1016/j.heares.2016.12.010.
Hind, S. E., Haines-Bazrafshan, R., Benton, C. L., Brassington, W., Towle, B., & Moore, D. R. (2011). Prevalence of clinical referrals having hearing thresholds within normal limits. International Journal of Audiology, 50, 708-716, https://doi.org/10.3109/14992027.2011.582049.
Johannesen, P. T., Buzo, B. C., & Lopez-Poveda, E. A. (2019). Evidence for age-related cochlear synaptopathy in humans unconnected to speech-in-noise intelligibility deficits. Hearing Research, 374, 35-48, https://doi.org/10.1016/j.heares.2019.01.017.
Johannesen, P. T., Leclere, T., Wijetillake, A., Segovia-Martínez, M., & Lopez-Poveda, E. A. (2022). Modeling temporal information encoding by the population of fibers in the healthy and synaptopathic auditory nerve. Hearing Research, 426, 108621, https://doi.org/10.1016/j.heares.2022.108621.
Johannesen, P. T. & Lopez-Poveda, E. A. (2021). Age-related central gain compensation for reduced auditory nerve output for people with normal audiograms, with and without tinnitus. iScience, 24, 102658, https://doi.org/10.1016/j.isci.2021.102658.
Keshishzadeh, S., Garrett, M., Vasilkov, V., & Verhulst, S. (2020). The derived-band envelope following response and its sensitivity to sensorineural hearing deficits. Hearing Research, 392, 107979, https://doi.org/10.1016/j.heares.2020.107979.
Keshishzadeh, S., Garrett, M., & Verhulst, S. (2021). Towards personalized auditory models: Predicting individual sensorineural hearing-loss profiles from recorded human auditory physiology. Trends in Hearing, 25, 233121652098840, https://doi.org/10.1177/2331216520988406.
Knipper, M., Dijk, P. V., Nunes, I., Rüttiger, L., & Zimmermann, U. (2013). Advances in the neurobiology of hearing disorders: Recent developments regarding the basis of tinnitus and hyperacusis. Progress in Neurobiology, 111, 17-33, https://doi.org/10.1016/j.pneurobio.2013.08.002.
Kujawa, S. G. & Liberman, M. C. (2009). Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. The Journal of Neuroscience, 29, 14077-14085, https://doi.org/10.1523/JNEUROSCI.2845-09.2009.
Kumar, G., Amen, F., & Roy, D. (2007). Normal hearing tests: is a further appointment really necessary? Journal of the Royal Society of Medicine, 100, 66-66, https://doi.org/10.1258/jrsm.100.2.66-a.
Lai, J., Sommer, A. L., & Bartlett, E. L. (2017). Age-related changes in envelope-following responses at equalized peripheral or central activation. Neurobiology of Aging, 58, 191-200, https://doi.org/10.1016/j.neurobiolaging.2017.06.013.
Leake, P. A., Akil, O., & Lang, H. (2020). Neurotrophin gene therapy to promote survival of spiral ganglion neurons after deafness. Hearing Research, 394, 107955, https://doi.org/10.1016/j.heares.2020.107955.
Le Prell, C. G., Siburt, H. W., Lobarinas, E., Griffiths, S. K., & Spankovich, C. (2018). No reliable association between recreational noise exposure and threshold sensitivity, distortion product otoacoustic emission amplitude, or word-in-noise performance in a college student population. Ear Hearing, 39, 1057-1074, https://doi.org/10.1097/AUD.0000000000000575.
Liberman, M. & Kiang, N. Y.-S. (1984). Single-neuron labeling and chronic cochlear pathology. iv. stereocilia damage and alterations in rate- and phase-level functions. Hearing Research, 16, 75-90, https://doi.org/10.1016/0378-5955(84)90026-1.
Liberman, M. C. (1978). Auditory-nerve response from cats raised in a low-noise chamber. The Journal of the Acoustical Society of America, 63, 442-455, https://doi.org/10.1121/1.381736.
Liberman, M. C. (1982). Single-neuron labeling in the cat auditory nerve. Science, 216, 1239-1241, https://doi.org/10.1126/science.7079757.
Liberman, M. C., Epstein, M. J., Cleveland, S. S., Wang, H., & Maison, S. F. (2016). Toward a differential diagnosis of hidden hearing loss in humans. PLOS ONE, 11, e0162726, https://doi.org/10.1371/journal.pone.0162726.
Liberman, M. C. & Kujawa, S. G. (2017). Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hearing Research, 349, 138-147, https://doi.org/10.1016/j.heares.2017.01.003.
Lin, H. W., Furman, A. C., Kujawa, S. G., & Liberman, M. C. (2011). Primary neural degeneration in the guinea pig cochlea after reversible noise-induced threshold shift. Journal of the Association for Research in Otolaryngology, 12, 605-616, https://doi.org/10.1007/s10162-011-0277-0.
Liu, J., Stohl, J., & Overath, T. (2024). Hidden hearing loss: Fifteen years at a glance. Hearing Research, 443, 108967, https://doi.org/10.1016/j.heares.2024.108967.
Liu, L., Wang, H., Shi, L., Almuklass, A., He, T., Aiken, S., Bance, M., Yin, S., & Wang, J. (2012). Silent damage of noise on cochlear afferent innervation in guinea pigs and the impact on temporal processing. PLoS ONE, 7, e49550, https://doi.org/10.1371/journal.pone.0049550.
Lobarinas, E., Salvi, R., & Ding, D. (2013). Insensitivity of the audiogram to carboplatin induced inner hair cell loss in chinchillas. Hearing Research, 302, 113-120, https://doi.org/10.1016/j.heares.2013.03.012.
Lobarinas, E., Spankovich, C., & Le Prell, C. G. (2017). Evidence of “hidden hearing loss” following noise exposures that produce robust tts and abr wave-i amplitude reductions. Hearing Research, 349, 155-163, https://doi.org/10.1016/j.heares.2016.12.009.
Lopez-Poveda, E. A. (2014). Why do I hear but not understand? Stochastic undersampling as a model of degraded neural encoding of speech. Frontiers in Neuroscience, 8, 348, https://doi.org/10.3389/fnins.2014.00348.
Lopez-Poveda, E. A. & Barrios, P. (2013). Perception of stochastically undersampled sound waveforms: a model of auditory deafferentation. Frontiers in Neuroscience, 7, 124, https://doi.org/10.3389/fnins.2013.00124.
Maele, T. V., Keshishzadeh, S., Poortere, N. D., Dhooge, I., Keppler, H., & Verhulst, S. (2021). The variability in potential biomarkers for cochlear synaptopathy after recreational noise exposure. Journal of Speech, Language, and Hearing Research, 64, 4964-4981, https://doi.org/10.1044/2021_JSLHR-21-00064.
Makary, C. A., Shin, J., Kujawa, S. G., Liberman, M. C., & Merchant, S. N. (2011). Age-related primary cochlear neuronal degeneration in human temporal bones. Journal of the Association for Research in Otolaryngology, 12, 711-717, https://doi.org/10.1007/s10162-011-0283-2.
Märcher-Rørsted, J., Encina-Llamas, G., Dau, T., Liberman, M. C., Wu, P., & Hjortkjær, J. (2022). Age- related reduction in frequency-following responses as a potential marker of cochlear neural degeneration. Hearing Research, 414, 108411, https://doi.org/10.1016/j.heares.2021.108411.
Marmel, F., Rodríguez-Mendoza, M. A., & Lopez-Poveda, E. A. (2015). Stochastic undersampling steepens auditory threshold/duration functions: implications for understanding auditory deafferentation and aging. Frontiers in aging neuroscience, 7, 63, https://doi.org/10.3389/fnagi.2015.00063.
Maxwell, K. S., Robinson, J. M., Hoffmann, I., Hou, H. J., Searchfield, G., Baguley, D. M., McMurry, G., Piu, F., & Anderson, J. J. (2021). Intratympanic administration of oto-313 reduces tinnitus in patients with moderate to severe, persistent tinnitus: A phase 1/2 study. Otology Neurotology, 42, e1625-e1633, https://doi.org/10.1097/MAO.0000000000003369.
Mehraei, G., Hickox, A. E., Bharadwaj, H. M., Goldberg, H., Verhulst, S., Liberman, M. C., & Shinn-Cunningham, B. G. (2016). Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. The Journal of Neuroscience, 36, 3755-3764, https://doi.org/10.1523/JNEUROSCI.4460-15.2016.
Mepani, A. M., Verhulst, S., Hancock, K. E., Garrett, M., Vasilkov, V., Bennett, K., de Gruttola, V., Liberman, M. C., & Maison, S. F. (2021). Envelope following responses predict speech-in-noise performance in normal- hearing listeners. Journal of Neurophysiology, 125, 1213-1222, https://doi.org/10.1152/jn.00620.2020.
Möhrle, D., Hofmeier, B., Amend, M., Wolpert, S., Ni, K., Bing, D., Klose, U., Pichler, B., Knipper, M., & Rüttiger, L. (2019). Enhanced central neural gain compensates acoustic trauma-induced cochlear impairment, but unlikely correlates with tinnitus and hyperacusis. Neuroscience, 407, 146-169, https://doi.org/10.1016/j.neuroscience.2018.12.038.
Möhrle, D., Ni, K., Varakina, K., Bing, D., Lee, S. C., Zimmermann, U., Knipper, M., & Rüttiger, L. (2016). Loss of auditory sensitivity from inner hair cell synaptopathy can be centrally compensated in the young but not old brain. Neurobiology of Aging, 44, 173-184, https://doi.org/10.1016/j.neurobiolaging.2016.05.001.
Parthasarathy, A., Bartlett, E. L., & Kujawa, S. G. (2019). Age-related changes in neural coding of envelope cues: Peripheral declines and central compensation. Neuroscience, 407, 21-31, https://doi.org/10.1016/j.neuroscience.2018.12.007.
Parthasarathy, A. & Kujawa, S. G. (2018). Synaptopathy in the aging cochlea: Characterizing early- neural deficits in auditory temporal envelope processing. The Journal of Neuroscience, 38, 7108-7119, https://doi.org/10.1523/JNEUROSCI.3240-17.2018.
Paul, B. T., Bruce, I. C., & Roberts, L. E. (2017). Evidence that hidden hearing loss underlies amplitude modulation encoding deficits in individuals with and without tinnitus. Hearing Research, 344, 170-182, https://doi.org/10.1016/j.heares.2016.11.010.
Peñuela, J. M., Oyarzabal, S. M., Batalla, F. N., López, R. H. S., & Herrero, T. V. (2022). Manual de audiología laboral. Lettera publicaciones. https://letterapublicaciones.com/producto/manual-de-audiologia-laboral/.
Prendergast, G., Guest, H., Munro, K. J., Kluk, K., Léger, A., Hall, D. A., Heinz, M. G., & Plack, C. J. (2017a). Effects of noise exposure on young adults with normal audiograms I: Electrophysiology. Hearing Research, 344, 68-81, https://doi.org/10.1016/j.heares.2016.10.028.
Prendergast, G., Millman, R. E., Guest, H., Munro, K. J., Kluk, K., Dewey, R. S., Hall, D. A., Heinz, M. G., & Plack, C. J. (2017b). Effects of noise exposure on young adults with normal audiograms II: Behavioral measures. Hearing Research, 356, 74-86, https://doi.org/10.1016/j.heares.2017.10.007.
Prendergast, G., Tu, W., Guest, H., Millman, R. E., Kluk, K., Couth, S., Munro, K. J., & Plack, C. J. (2018). Supra-threshold auditory brainstem response amplitudes in humans: Test-retest reliability, electrode montage and noise exposure. Hearing Research, 364, 38-47, https://doi.org/10.1016/j.heares.2018.04.002.
Ridley, C. L., Kopun, J. G., Neely, S. T., Gorga, M. P., & Rasetshwane, D. M. (2018). Using thresholds in noise to identify hidden hearing loss in humans. Ear Hearing, 39, 829-844, https://doi.org/10.1097/AUD.0000000000000543.
Roberts, L. E., Paul, B. T., & Bruce, I. C. (2018). Erratum and comment: Envelope following responses in normal hearing and in tinnitus. Hearing Research, 361, 157-158, https://doi.org/10.1016/j.heares.2018.01.011.
Rumschlag, J. A., McClaskey, C. M., Dias, J. W., Kerouac, L. B., Noble, K. v., Panganiban, C., Lang, H., & Harris, K. C. (2022). Age-related central gain with degraded neural synchrony in the auditory brainstem of mice and humans. Neurobiology of Aging, 115, 50-59, https://doi.org/10.1016/j.neurobiolaging.2022.03.014.
Ryan, A. & Dallos, P. (1975). Effect of absence of cochlear outer hair cells on behavioural auditory threshold. Nature, 253, 44-6, https://doi.org/10.1038/253044a0.
Salvi, R., Sun, W., Ding, D., Chen, G.-D., Lobarinas, E., Wang, J., Radziwon, K., & Auerbach, B. D. (2017). Inner hair cell loss disrupts hearing and cochlear function leading to sensory deprivation and enhanced central auditory gain. Frontiers in Neuroscience, 10, 1-14, https://doi.org/10.3389/fnins.2016.00621.
Saunders, G. H. & Haggard, M. P. (1989). The clinical assessment of obscure auditory dysfunction— 1. Auditory and psychological factors. Ear and Hearing, 10, 200-208, https://doi.org/10.1097/00003446-198906000-00011.
Schaette, R. (2014). Tinnitus in men, mice (as well as other rodents), and machines. Hearing Research, 311, 63-71, https://doi.org/10.1016/j.heares.2013.12.004.
Schaette, R. & McAlpine, D. (2011). Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. The Journal of Neuroscience, 31, 13452-13457, https://doi.org/10.1523/JNEUROSCI.2156-11.2011.
Schuknecht, H. F. & Woellner, R. C. (1955). An experimental and clinical study of deafness from lesions of the cochlear nerve. The Journal of Laryngology Otology, 69, 75-97, https://doi.org/10.1017/S0022215100050465.
Sergeyenko, Y., Lall, K., Liberman, M. C., & Kujawa, S. G. (2013). Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. The Journal of Neuroscience, 33, 13686-13694, https://doi.org/10.1523/JNEUROSCI.1783-13.2013.
Sheppard, A., Liu, X., Ding, D., & Salvi, R. (2018). Auditory central gain compensates for changes in cochlear output after prolonged low-level noise exposure. Neuroscience Letters, 687, 183-188, https://doi.org/10.1016/j.neulet.2018.09.054.
Shi, L., Liu, L., He, T., Guo, X., Yu, Z., Yin, S., & Wang, J. (2013). Ribbon synapse plasticity in the cochleae of guinea pigs after noise-induced silent damage. PLoS ONE, 8, e81566, https://doi.org/10.1371/journal.pone.0081566.
Skoe, E. & Tufts, J. (2018). Evidence of noise-induced subclinical hearing loss using auditory brainstem responses and objective measures of noise exposure in humans. Hearing Research, 361, 80-91, https://doi.org/10.1016/j.heares.2018.01.005.
Spankovich, C., Prell, C. G. L., Lobarinas, E., & Hood, L. J. (2017). Noise history and auditory function in young adults with and without type 1 diabetes mellitus. Ear Hearing, 38, 724-735, https://doi.org/10.1097/AUD.0000000000000457.
Stamper, G. C. & Johnson, T. A. (2015). Auditory function in normal-hearing, noise-exposed human ears. Ear Hearing, 36, 172-184, https://doi.org/10.1097/AUD.0000000000000107.
Suthakar, K. & Liberman, M. C. (2021). Auditory-nerve responses in mice with noise-induced cochlear synaptopathy. Journal of Neurophysiology, 126, 2027-2038, https://doi.org/10.1152/jn.00342.2021.
Suzuki, J., Corfas, G., & Liberman, M. C. (2016). Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Scientific Reports, 6, 24907, https://doi.org/10.1038/srep24907.
Taberner, A. M. & Liberman, M. C. (2005). Response properties of single auditory nerve fibers in the mouse. Journal of Neurophysiology, 93, 557-569, https://doi.org/10.1152/jn.00574.2004.
Temboury-Gutierrez, M., Encina-Llamas, G., & Dau, T. (2024a). Predicting early auditory evoked potentials using a computational model of auditory-nerve processing. The Journal of the Acoustical Society of America, 155, 1799-1812, https://doi.org/10.1121/10.0025136.
Temboury-Gutierrez, M., Märcher-Rørsted, J., Bille, M., Yde, J., Encina-Llamas, G., Hjortkjær, J., & Dau, T. (2024b). Electrocochleographic frequency-following responses as a potential marker of age-related cochlear neural degeneration. Hearing Research, 446, 109005. https://doi.org/10.1016/j.heares.2024.109005
Tremblay, K. L., Pinto, A., Fischer, M. E., Klein, B. E. K., Klein, R., Levy, S., Tweed, T. S., & Cruickshanks, K. J. (2015). Self-reported hearing difficulties among adults with normal audiograms. Ear Hearing, 36, e290-e299, https://doi.org/10.1097/AUD.0000000000000195.
Valderrama, J. T., Beach, E. F., Yeend, I., Sharma, M., Dun, B. V., & Dillon, H. (2018). Effects of lifetime noise exposure on the middle-age human auditory brainstem response, tinnitus and speech-in-noise intelligibility. Hearing Research, 365, 36-48, https://doi.org/10.1016/j.heares.2018.06.003.
Valderrama, J. T., de la Torre, A., & McAlpine, D. (2022). The hunt for hidden hearing loss in humans: From preclinical studies to effective interventions. Frontiers in Neuroscience, 16, https://doi.org/10.3389/fnins.2022.1000304.
Valero, M. D., Burton, J. A., Hauser, S. N., Hackett, T. A., Ramachandran, R., & Liberman, M. C. (2017). Noise-induced cochlear synaptopathy in rhesus monkeys (macaca mulatta). Hearing research, 353, 213-223, https://doi.org/10.1016/j.heares.2017.07.003.
Vasilkov, V., Garrett, M., Mauermann, M., & Verhulst, S. (2021). Enhancing the sensitivity of the envelope-following response for cochlear synaptopathy screening in humans: The role of stimulus envelope. Hearing Research, 400, 108132, https://doi.org/10.1016/j.heares.2020.108132.
Verhulst, S., Altoe, A., & Vasilkov, V. (2018). Computational modeling of the human auditory periphery: Auditory-nerve responses, evoked potentials and hearing loss. Hearing Research, 360, 55-75, https://doi.org/10.1016/j.heares.2017.12.018.
Viana, L. M., O’Malley, J. T., Burgess, B. J., Jones, D. D., Oliveira, C. A. C. P., Santos, F., Merchant, S. N., Liberman, L. D., & Liberman, M. C. (2015). Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hearing Research, 327, 78-88, https://doi.org/10.1016/j.heares.2015.04.014.
Wojtczak, M., Beim, J. A., & Oxenham, A. J. (2017). Weak middle-ear-muscle reflex in humans with noise-induced tinnitus and normal hearing may reflect cochlear synaptopathy. eneuro, 4, ENEURO.0363- 17.2017, https://doi.org/10.1523/ENEURO.0363-17.2017.
Wu, P., Liberman, L. D., Bennett, K., de Gruttola, V., O’Malley, J. T., & Liberman, M. (2019). Primary neural degeneration in the human cochlea: Evidence for hidden hearing loss in the aging ear. Neuroscience, 407, 8-20, https://doi.org/10.1016/j.neuroscience.2018.07.053.
Wu, P., O’Malley, J. T., de Gruttola, V., & Liberman, M. C. (2020). Age-related hearing loss is dominated by damage to inner ear sensory cells, not the cellular battery that powers them. The Journal of Neuroscience, 40, 6357-6366, https://doi.org/10.1523/JNEUROSCI.0937-20.2020.
Wu, P., O’Malley, J. T., de Gruttola, V., & Liberman, M. C. (2021). Primary neural degeneration in noise-exposed human cochleas: Correlations with outer hair cell loss and word-discrimination scores. The Journal of Neuroscience, 41, 4439-4447, https://doi.org/10.1523/JNEUROSCI.3238-20.2021.
Yeend, I., Beach, E. F., Sharma, M., & Dillon, H. (2017). The effects of noise exposure and musical training on suprathreshold auditory processing and speech perception in noise. Hearing Research, 353, 224-236, https://doi.org/10.1016/j.heares.2017.07.006.
Zan, P., Presacco, A., Anderson, S., & Simon, J. Z. (2020). Exaggerated cortical representation of speech in older listeners: mutual information analysis. Journal of Neurophysiology, 124, 1152-1164, https://doi.org/10.1152/jn.00002.2020.
Conflict of interest
The authors declare no conflict of interest.
Funds
This work has been funded by a grant from the GN Foundation (ref. 237) as part of the AudPhen (Auditory Phenotypes in Denmark) at the Rigshospitalet hospital in Copenhagen (Denmark).
How to cite
Encina-Llamas, G. (2024).
Cochlear synaptopathy. Auditio, 8, e103.
https://doi.org/10.51445/sja.auditio.vol8.2024.103
Correspondence
Gerard Encina-Llamas
Email: gerard.encina@umedicina.cat
Editorial Office
Copyeditor: Tomás Pérez Pazos
Translation: Emma Goldsmith
Production: Glaux Publicaciones Académicas
